

| ||
| ||
| ||
| 160 |
| x |
| 112 |
| y |
| 3��44 |
| 66g |
| 100g-80g |
| 100g |


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ��ɽ��������������һѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�������
����Fe��Fe2O3�Ĺ������Ϊ�˷����������Fe��Fe2O3�ĺ��������������ʵ�鷽����
��ʵ�����ݡ�ʵ�鹲��¼������ʵ�����ݣ��ڢ��飬������ȫ���պ�ʯ��ˮ��Һ��������66g���ڢ��飬��ȫ��Ӧ����ȴ�����ʣ����������ΪWg��
Fe2O3��CO��Ӧ����ʽ�ǣ�Fe2O3 + 3CO 2Fe + 3CO2
2Fe + 3CO2
����ʵ����Ƽ��й����ݽ��з��������
��1����������������� g��
��2���������Fe���ʵ���������Ϊ���٣���д��������̣�
��3���ڢ�������W�� g��д������������
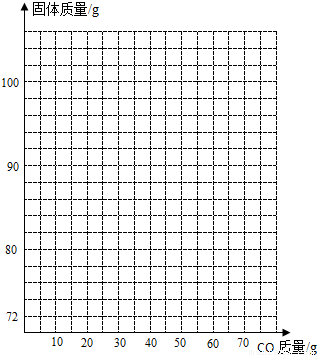
��4������100g Fe��Fe2O3�Ĺ��������г������ϵ�ͨ��CO�����㻭������������ͨ��CO�����Ĺ�ϵͼ���ڴ������������ͼ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����л����ؿ�̶��ѧ���꼶���£���ĩ��ѧģ���Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ɽ�����������꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
 2Fe+3CO2
2Fe+3CO2�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com