£Ø2006?øß“¾ĻŲ¶žÄ££©ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬ÓÉÓŚÓėŃŅŹÆ”¢“óĘųŗĶÉśĪļµÄĻą»„×÷ÓĆ£¬ŗ£Ė®ÖŠČܽāŗĶŠüø”ÓŠ“óĮæµÄĪŽ»śĪļŗĶÓŠ»śĪļ£¬×ܼĘ80¶ąÖÖŌŖĖŲ£¬¾ßÓŠ¾Ž“óµÄæŖ·¢Ē±ÄÜ£®
£Ø1£©ĄūÓĆĢ«ŃōÄÜÕō·¢ŗ£Ė®µĆµ½µĖ®µÄŹ¾ŅāĶ¼£¬ÕāŅ»¹ż³ĢŹĒ
ĪļĄķ
ĪļĄķ
±ä»Æ£ØĢī”°ĪļĄķ”±»ņ”°»Æѧ”±£©£®
£Ø2£©ČÕɹ·ØĄ“ÅØĖõŗ£Ė®£¬“żŃĪĖ®Õō·¢“ļµ½Ņ»¶ØÅضČŗ󣬾ĶÓŠ¾§ĢåĀżĀżĪö³ö£¬½«ĘäĄĢ³ö¶Ń³ÉŃĪŪē£¬Į÷³öµÄÄøŅŗ¾ĶŹĒŃĪĀ±£Ø»ņ³ĘĀ±Ė®£©£¬æÉŅŌÓĆĄ“”°µć”±¶¹øÆ£¬Ź¹¶¹½¬ÖŠµÄ
µ°°×ÖŹ
µ°°×ÖŹ
Äż¾Ū£®
£Ø3£©ŃĪŪēµÄŹ³ŃĪŹĒ“ÖŃĪ£¬Ķعż·ÖĄė”¢Ģį“棬æÉŅŌµĆµ½¾«ŃĪŗĶ²»Ķ¬µÄ²śĘ·£®ÄæĒ°ŹĄ½ēÉĻ60%µÄĆ¾¾ĶŹĒ“Óŗ£Ė®ÖŠĢįČ”µÄ£¬Ö÷ŅŖ²½ÖčČēĻĀ£ŗ

¢ŁĪŖĮĖŹ¹MgCl
2×Ŗ»ÆĪŖ Mg£ØOH£©
2£¬ŹŌ¼Į¢ŁæÉŅŌŃ”ÓĆ
NaOH
NaOH
£¬ŅŖŹ¹MgCl
2ĶźČ«×Ŗ»ÆĪŖ³Įµķ£¬¼ÓČėŹŌ¼Į¢ŁµÄĮæÓ¦
¹żĮæ
¹żĮæ
£»ŹŌ¼Į¢ŚæÉŅŌŃ”ÓĆ
Ļ”HCl
Ļ”HCl
¢Ś¼ÓČėŹŌ¼Į¢Łŗó£¬Äܹ»·ÖĄėµĆµ½Mg£ØOH£©
2³ĮµķµÄ·½·ØŹĒ
¹żĀĖ
¹żĀĖ
£»
¢ŪĪŽĖ®MgCl
2ŌŚČŪȌדĢ¬ĻĀ£¬Ķصēŗó»į²śÉśĆ¾ŗĶĀČĘų£¬Š“³öøĆ·“Ó¦»Æѧ·½³ĢŹ½
£Ø4£©Ķ¬Ń§ĆĒŌŚ×öĆ¾ĢõŌŚæÕĘųÖŠČ¼ÉÕŹµŃ鏱·¢ĻÖĆ¾Ģõ±ķĆę±»Ņ»²ćŗŚÉ«µÄĪļÖŹAĖłø²øĒ£¬ÕāŅ»²ćŗŚÉ«µÄĪļÖŹŹĒŹ²Ć“ÄŲ£æÄ³ŃŠ¾æŠŌѧĻ°Š”×éŹÕ¼ÆŗŚÉ«ĪļÖŹA£¬¶ŌĘä³É·Ö½ųŠŠŹµŃéĢ½¾æ£¬ŹµŃé¼ĒĀ¼ČēĶ¼ĖłŹ¾£®

ĒėÄćøł¾ŻŹµŃéĶʶĻ£ŗ
£Ø1£©AÖŠŗ¬ÓŠµÄŌ×ÓĶÅŹĒ
CO32-
CO32-
£ØŠ“Ąė×Ó·ūŗÅ£©£®
£Ø2£©·“Ó¦¢ŚµÄ»ł±¾·“Ó¦ĄąŠĶŹĒ
·Ö½ā
·Ö½ā
·“Ó¦£ØŃ”Ģī»ÆŗĻ”¢·Ö½ā”¢ÖĆ»»£©£®
£Ø3£©Š“³öŹµŃéÖŠÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ¢Ü
CO2+Ca£ØOH£©2ØTCaCO3”ż+H20
CO2+Ca£ØOH£©2ØTCaCO3”ż+H20
£®
£Ø4£©øł¾ŻŹµŃé½į¹ūĶʶĻAÖŠæĻ¶Øŗ¬ÓŠµÄŌŖĖŲÓŠ£ŗ
CӢHӢOӢMg
CӢHӢOӢMg
£®
£Ø5£©°×É«·ŪÄ©DæÉÄÜŹĒ
¼īŹ½Ģ¼ĖįĆ¾
¼īŹ½Ģ¼ĖįĆ¾
£®

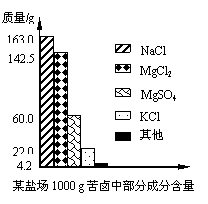
 ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬¾ßÓŠŹ®·Ö¾Ž“óµÄæŖ·¢Ē±Į¦£®æąĀ±ŹĒŗ£Ė®ĢįČ”Ź³ŃĪŗóµÄ²ŠŅŗ£¬ĄūÓĆæąĀ±æÉŅŌÖĘµĆ½šŹōĆ¾µČ»Æ¹¤ŌĮĻ£®ČēĶ¼ŹĒijŃĪ³”æąĀ±ÖŠ²æ·Ö³É·Öŗ¬ĮæÖłŠĪĶ¼£®ŹŌ¼ĘĖć£ŗ
ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬¾ßÓŠŹ®·Ö¾Ž“óµÄæŖ·¢Ē±Į¦£®æąĀ±ŹĒŗ£Ė®ĢįČ”Ź³ŃĪŗóµÄ²ŠŅŗ£¬ĄūÓĆæąĀ±æÉŅŌÖĘµĆ½šŹōĆ¾µČ»Æ¹¤ŌĮĻ£®ČēĶ¼ŹĒijŃĪ³”æąĀ±ÖŠ²æ·Ö³É·Öŗ¬ĮæÖłŠĪĶ¼£®ŹŌ¼ĘĖć£ŗ

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
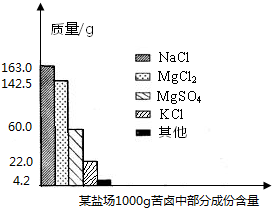
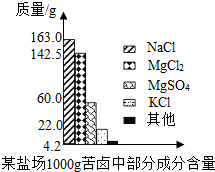 ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬¾ßÓŠŹ®·Ö¾Ž“óµÄæŖ·¢Ē±Į¦£®æąĀ±ŹĒŗ£Ė®ĢįČ”Ź³ŃĪŗóµÄ²ŠŅŗ£¬ĄūÓĆæąĀ±æÉŅŌÖĘµĆ½šŹōĆ¾µČ»Æ¹¤ŌĮĻ£®ĻĀĶ¼ŹĒĪŅŹŠÄ³ŃĪ³”æąĀ±ÖŠ²æ·Ö³É·Öŗ¬ĮæÖłŠĪĶ¼£®ŹŌ¼ĘĖć£ŗ
ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬¾ßÓŠŹ®·Ö¾Ž“óµÄæŖ·¢Ē±Į¦£®æąĀ±ŹĒŗ£Ė®ĢįČ”Ź³ŃĪŗóµÄ²ŠŅŗ£¬ĄūÓĆæąĀ±æÉŅŌÖĘµĆ½šŹōĆ¾µČ»Æ¹¤ŌĮĻ£®ĻĀĶ¼ŹĒĪŅŹŠÄ³ŃĪ³”æąĀ±ÖŠ²æ·Ö³É·Öŗ¬ĮæÖłŠĪĶ¼£®ŹŌ¼ĘĖć£ŗ

 (2)½«ÉĻŹö³ĮµķĶźČ«×Ŗ»ÆĪŖĪŽĖ®MgCl2£¬²¢ŌŚČŪȌדĢ¬ĻĀ½ųŠŠµē½ā£¬æÉµĆ½šŹōĆ¾¶ąÉŁæĖ£æĀČĘų¶ąÉŁÉż£ØøĆĢõ¼žĻĀĀČĘųĆܶČĪŖ3.0 g/L£©£æ
(2)½«ÉĻŹö³ĮµķĶźČ«×Ŗ»ÆĪŖĪŽĖ®MgCl2£¬²¢ŌŚČŪȌדĢ¬ĻĀ½ųŠŠµē½ā£¬æÉµĆ½šŹōĆ¾¶ąÉŁæĖ£æĀČĘų¶ąÉŁÉż£ØøĆĢõ¼žĻĀĀČĘųĆܶČĪŖ3.0 g/L£©£æ