
 ijƷ�ƻ��ʵı�ǩ��ͼ��ʾ��С��ͨ��ʵ��ⶨ�û��ʱ�ǩ�ĺ������Ƿ���ʵ������������82g�û�����Ʒ�����ձ��У�����NaOH��Һ���ⶨ�����NaOH��Һ�������ձ��������������±���ʾ����������������Ʒ�Ӧ�Ļ�ѧ����ʽΪNH4NO3+NaOH=NaNO3+H2O+NH3���������������������������Ʋ���Ӧ��
ijƷ�ƻ��ʵı�ǩ��ͼ��ʾ��С��ͨ��ʵ��ⶨ�û��ʱ�ǩ�ĺ������Ƿ���ʵ������������82g�û�����Ʒ�����ձ��У�����NaOH��Һ���ⶨ�����NaOH��Һ�������ձ��������������±���ʾ����������������Ʒ�Ӧ�Ļ�ѧ����ʽΪNH4NO3+NaOH=NaNO3+H2O+NH3���������������������������Ʋ���Ӧ��| ����NaOH��Һ����/g | 30 | 60 | 90 | 120 |
| �ձ������ʵ�����/g | 106.9 | 131.8 | 156.7 | 185 |
���� ����ͼ�����ݣ�ǰ����ÿ����30g����������Һ�õ������嶼��5.1g�������뵽���ĸ�30g����������Һʱ��ֻ�õ�1.7g������˵����ʱ������Ѿ���ȫ��Ӧ�����Դ��������仯�õ��������������������ݰ����������Ͷ�Ӧ�Ļ�ѧ����ʽ��������淋�����Ȼ�����㵪Ԫ�ص��������߸��ݹ�ϵʽ���㵪Ԫ�ص�������
��� �⣺����ͼ�����ݣ�ǰ����ÿ����30g����������Һ�õ������嶼��5.1g�������뵽���ĸ�30g����������Һʱ��ֻ�õ�1.7g������˵����ʱ������Ѿ���ȫ��Ӧ�����������غ㶨�ɿɵã����ɵİ���������Ϊ82g+120g-185g=17g
����һ��
������淋�����Ϊx
NH4NO3+NaOH=NaNO3+H2O+NH3��
80 17
x 17g
$\frac{80}{17}$=$\frac{x}{17g}$
x=80g
��������Ԫ�ص�����Ϊ80g��$\frac{14��2}{14��2+1��4+16��3}$��100%=28g
�û��ʵĺ�������$\frac{28g}{82g}$��100%��34.1%����ʵ�ʱ�ǩ����
���������ù�ϵʽ����
�赪Ԫ�ص�����Ϊy
2N--------------NH3��
28 17
y 17g
$\frac{28}{17g}$=$\frac{y}{17g}$
y=28g
�û��ʵĺ�������$\frac{28g}{82g}$��100%��34.1%����ʵ�ʱ�ǩ����
ͨ�������֪�������еĵ�Ԫ��������������е�笠���������������е�Ԫ�ص�ȫ�������Բ��ܸ��ݰ����еĵ�Ԫ�����е�Ԫ�ص�����������
ͬʱ���ڰ��������ܽ���ˮ�����Ե������ɵİ���������ȫ�ݳ�������������Һ�У����µõ��Ľ���ȸû���ʵ�ʵĺ�������ƫС��
�𣺣�1��82g����������NH4NO3ȫ����NaOH��Һ��Ӧʱ�����ɵİ��������� 17g��
��2��ͨ�������֪�û��ʱ�ǩ�ϵĺ����� ����ʵ�ʣ�
��3��С��ͬѧ��Ϊ���÷�Ӧ�����İ����е�Ԫ�ص�����Ҳ����ֱ����������е�Ԫ�ص����������� С�������ԭ�� �����еĵ�Ԫ��������������е�笠���������������е�Ԫ�ص�ȫ����
��4��СƼͬѧ��ΪС��ͬѧ�õ��Ľ���ȸû���ʵ�ʵĺ�������ƫС��СƼͬѧ��ΪƫС�������� ���������ܽ���ˮ��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������


 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | l%KOH | B�� | 10% KOH | C�� | 1%HC1 | D�� | 10% HC1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
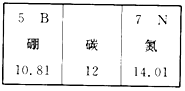
| A�� | ̼Ԫ�ض�Ӧԭ�ӵ�������Ϊ12 | |
| B�� | ̼Ԫ�����ڷǽ���Ԫ�� | |
| C�� | ����̼Ԫ�صĻ����ﶼ���л������� | |
| D�� | ��̼Ԫ����ɵĵ��ʻ�ѧ���ʻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
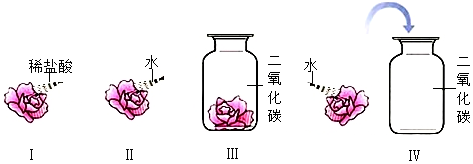
 ��ͼ��ijƷ�ơ��߸�Ƭ��˵�����һ���֣������˵��������ݻش��������⣺
��ͼ��ijƷ�ơ��߸�Ƭ��˵�����һ���֣������˵��������ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �÷�Ӧ�з��Ӻ�ԭ�ӵ���������˸ı� | |
| B�� | ���ʱ��Ļ�ѧʽ�� NH3 | |
| C�� | �ס��������ʲμӷ�Ӧ��������Ϊ 1��3 | |
| D�� | ͼʾ��Ӧ���ڷֽⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
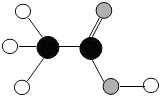 ʳ���dz����г��õĵ�ζƷ��������Ҫ�ɷ������ᡢ������ӵ�ģ����ͼ��ʾ�����С�
ʳ���dz����г��õĵ�ζƷ��������Ҫ�ɷ������ᡢ������ӵ�ģ����ͼ��ʾ�����С� ������һ��̼ԭ�ӣ���
������һ��̼ԭ�ӣ���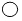 ������һ����ԭ�ӣ���
������һ����ԭ�ӣ��� ������һ����ԭ�ӣ�����˵������ȷ���ǣ�������
������һ����ԭ�ӣ�����˵������ȷ���ǣ�������| A�� | ������̼Ԫ�ص���������Ϊ60% | |
| B�� | �������Է�������Ϊ60 | |
| C�� | ������һ�ֻ����� | |
| D�� | ��������е�̼ԭ�ӡ���ԭ�ӡ���ԭ�ӵĸ�����Ϊ1��2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 g | B�� | 2 g | C�� | 3.2 g | D�� | 4 g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com