
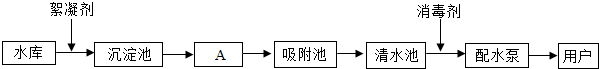
��ͼ��ˮ������ˮ���е�ˮ��������ˮ����Ҫ���̣�
��ش��������⣺

��1��ˮ����Ⱦ�������أ����ˮ����Ⱦ����Դ��Ҫ�� ��ũҵ��Ⱦ��������Ⱦ��
ȡˮ����������������������� ��
��2��A����ͨ�� ����������ƣ���ȥˮ�еĹ������ʣ���ʵ���ҽ��иò������õ��IJ����������ձ����������� ��
��3����������ʹ�û���̿����������_________�ԣ���ȥˮ���е�ɫ�غ���ζ��
��4��������ˮ��һ������Ϊ����ˮ��ʵ�鷽���� ��ʵ������ȡ����ˮ��������ƿ�ͨ��Ҫ���뼸����ʯ�����Ƭ�������� ��
��5���������������Χͣˮ��Ϊ��ֹ����������Ⱦ�������ʱˮԴ��ˮ���������ã������д�ʩ��ˮ�Ϻ�����˳��Ϊ ������ţ���
�ټ������ ������ �۹��� ����Ȼ����
��1����ҵ��Ⱦ ���������ʡ�
��2�����ˣ�©�� ��
��3�� ����
��4�� ���� �� ��ֹ���С�
��5���ܢۢڢ�
��������
�����������1����ҵ��ũҵ��������ˮ������Ⱦ����������ˮ��������״���������ˮ��С�������ʱ�ɴ�������ʴӶ�ʹ֮������
��2��ʵ�ֹ�Һ��������������ˣ��õ�����������©����
��3������̿���ɶ�ף����кܺõ������ԡ�
��4���������ˮֻ��������ķ������ӷ�ʯ�������Ƿ�ֹ���У������ռ���������Һ�л��зе㲻ͬ�����ʡ�
��5����ˮ˳��Ӧ�������̶��ɵ͵���ʹ�ã�����Ч����������ʹ�á��ʴ�Ϊ�ܢۢڢ�
���㣺ˮ�ľ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
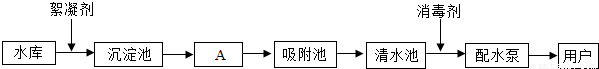
 ��ͼ��ˮ������ˮ���е�ˮ��������ˮ����Ҫ���̣�
��ͼ��ˮ������ˮ���е�ˮ��������ˮ����Ҫ���̣�
��ش��������⣺
��1��ˮ���е�ˮ���� ������������ ����
��2����A����ͨ�� ����������ƣ���ȥˮ�еĹ������ʡ�
�ڶ������ȣ�ClO2����һ�ָ�Ч����������������Ԫ�صĻ��ϼ�Ϊ �� �����������������ƣ�NaClO2����Ӧ��ȡClO2�ķ�Ӧ����ʽΪ��Cl2+2NaClO2==2ClO2+2X��
X�Ļ�ѧʽΪ ��
�۹��ҹ涨������ˮPH��ΧΪ6.5-8.5��Ϊ�˲ⶨ��������ˮ�Ƿ�ﵽ��һ�������� �����м�⡣
��3������ˮ��ͨ��������Cl-��������ˮ���еμ�����ϡ����� �� ���飬������
����˵��ˮ�к���Cl-��ˮ�е�Ca2+��Mg2+���Ⱥ�ת��Ϊ��������ͨ����˵��ˮ����ˮ������Ҫ�ɷ�Ϊ ��
��4��������ˮ��һ������Ϊ����ˮ��ʵ�鷽���� ��ʵ������ȡ����ˮ��������ƿ�ͨ��Ҫ���뼸����ʯ�����Ƭ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�꽭��ʡ�γ����п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com