

���� ����A��B��C��D��E��F�dz��л�ѧ�г��������ʣ�D�ǵ��ʣ���D��Ŀǰ�����������ߵĽ���������D��������C�ǵ���������Һ̬���������C����ˮ������ת���ɺ�ɫ����B������B��������������A���л�ԭ�ԣ�����A������������ɫ����E������ˮ������E����������ͭ�����ݸ��ֽⷴӦ��ԭ����F�����ڸ�������������GΪ��ɫ��Һ������F�����������ƣ�G��������ͭ��Ȼ���Ƴ������ʴ���ת����ϵ����֤���ɣ�
��� �⣺��1��A��B��C��D��E��F�dz��л�ѧ�г��������ʣ�D�ǵ��ʣ���D��Ŀǰ�����������ߵĽ���������D��������C�ǵ���������Һ̬���������C����ˮ������ת���ɺ�ɫ����B������B��������������A���л�ԭ�ԣ�����A������������ɫ����E������ˮ������E����������ͭ�����ݸ��ֽⷴӦ��ԭ����F�����ڸ�������������GΪ��ɫ��Һ������F�����������ƣ�G��������ͭ��������֤���Ƶ���ȷ������A��H2��B��Fe2O3��C��H2O��E��Cu��OH��2��
��2����Ӧ����δ����û�������ķ�ʽ��A��Ϊ����Դ��һ���ŵ�ȼ�ղ�����ˮ������Ⱦ��
��3����Ӧ��������������ͭ�ڸ��µ���������������ˮ����ѧ����ʽΪ��3H2+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3H2O����Ӧ�����������ƺ�����ͭ��Ӧ����������ͭ����������ƣ���ѧ����ʽΪ��Ca��OH��2+CuSO4=Cu��OH��2��+CaSO4��
�ʴ�Ϊ����1��H2��Fe2O3��H2O��Cu��OH��2��
��2��ȼ�ղ�����ˮ������Ⱦ��
��3��3H2+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3H2O��Ca��OH��2+CuSO4=Cu��OH��2��+CaSO4��
���� �ڽ������ʱ�����Ƚ������������������Ƴ���Ȼ�����Ƴ������ʺ����е�ת����ϵ�Ƶ�ʣ������ʣ�����Ƴ��ĸ������ʴ���ת����ϵ�н�����֤���ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
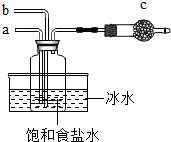 ��Ȼ����������̼�����������壬�ֶ��ǻ���ԭ�ϣ�
��Ȼ����������̼�����������壬�ֶ��ǻ���ԭ�ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ù�� | B�� | ѹե����֭ | ||
| C�� | ���ս� | D�� | ���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ��Ŀ�� | ʵ����� |
| A | ��Ӳˮת������ˮ | ��Ӳˮ�м��������������� |
| B | ��ȥCaO�е�CaCO3 | ����������ˮ������ܽ⣬���� |
| C | ��������狀����� | ����ʯ�ҷۡ���ĥ������ζ |
| D | ��ȥϡ�����л��е�����BaCl2��Һ | ����������CuSO4��Һ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10% | B�� | 20% | C�� | 42% | D�� | 50% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ������ | C�� | ̫���� | D�� | ú |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com