
�ܽ���ǽ����Һ����������Ҫ���ݡ�
I.�����ܽ�����߽����������: (M��N�������ᾧˮ)
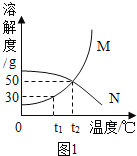
��1��t1��ʱ����20g M����50gˮ�У�����ܽ⣬�γ�_________(����͡������͡�)��Һ����Һ������Ϊ_____________g.�����¶Ȳ��䣬�����Һ���ټ���10gˮ��ֽ��裬��Һ����������������_______(��������С�����䡱) ;
��2��M�����к�������N���ʣ�����__________�����ᴿM����(����½ᾧ���������ᾧ��) ;
��3��t2��ʱ����25gN����50gˮ�У���ȫ�ܽ⡣������߸���Һ���������������������������____________��
II.���ݱ�����ʵ������:
�¶�/��C | 20 | 30 | 50 | 80 | 90 | |
�ܽ��/g | KNO3 | 31.6 | 45.8 | 85.5 | 100 | 169 |
K2CO3 | 110 | 114 | 121 | 126 | 139 |
ijKNO3��Ʒ�к�������K2CO3,���ᴿ������ͼ2(����������ˮ������û�б仯):
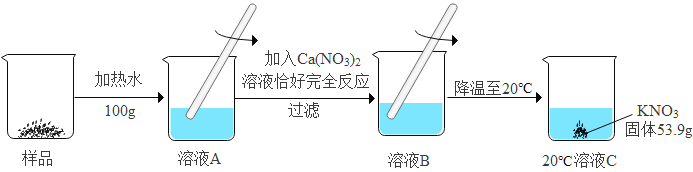
��1��ͼ2����ҺC��__________(����͡������͡�)��Һ; .
��2����Ʒ�м�Ԫ�ص�����Ϊ__________g (�����������).
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2019���Ĵ�ʡüɽ���������п�ģ�⻯ѧ�Ծ� ���ͣ������
��ѧԴ�����Ҳ���������
��1������ʱ�������Ż��ù��Ǹ��������ԭ����_____��
��2��Ѫ�쵰������_____��ϵ���ú���ж���
��3��üɽ������������ˮ����Ӳˮ�������г���_____�ķ�������ˮ��Ӳ�ȡ�
��4������ˮƿ��ʱ����ˮ���Զ��������˵��������ܽ����ѹǿ��С��_____�������С������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2018���������п���ģ��ѧ�Ծ� ���ͣ���ѡ��
��ˮ����Ҫ����Դ�����й��ں�ˮ�ı仯�����ڻ�ѧ�仯���ǣ�������
A.��ˮɹ�� B.��ˮ�ơ��
C.��ˮ��þ D.��ȥ��ˮ�е��Ȼ�þ�ȿ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2019��ɽ��ʡ�������������п���ģ��ѧ�Ծ� ���ͣ�������
����ʹ����ˮ���ײ��в�ˮ������Ҫ�ɷ�ΪCaCO3��Mg(OH)2��ijѧУ��ѧ�о�С���ͬѧͨ��ʵ��ⶨˮ����CaCO3�ĺ�������������ϡ������뵽200gˮ���У��Ѳ�����CO2������������NaOH��Һ���գ�ͬʱ����3min����NaOH��Һ���ӵ�������������±�:
ʱ��/s | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
����/g | 0 | 30 | 50 | 60 | 66 | 66 | 66 |
(1)д��CO2��������NaOH��Һ����ʱ�������Ļ�ѧ��Ӧ����ʽ_______________��
(2)�ӱ����п��Կ�����200gˮ�������ᷴӦ���ɵ�CO2�����___________g��
(3)����ˮ����̼��Ƶ�����������д���������_________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2019��ɽ��ʡ�������������п���ģ��ѧ�Ծ� ���ͣ������
�ܽ�������dz��л�ѧ�����ĸ����ش��������⡣
I.�±���NaC1��KNO3�ڲ�ͬ�¶��µ��ܽ��:
�¶�/�� | 0 | 20 | 40 | 60 | 80 | |
�ܽ��/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 |
(1)Ҫ�Ƚ�NaC1��KNO3��ˮ�е��ܽ���������Ҫ���Ƶı�����ˮ��������_____________________��
(2)��KNO3��Һ�л�ȡ�侧�����˵ķ�����_____________________��
(3)20��ʱ����ȡNaC1��KNO3����3.5g����10gˮ�У�����ܽ��ﵽ����״̬����___��Һ��
(4)�������������������л��Ƴ�NaCl��KNO3���ܽ�����ߣ��������ߵĽ������Ӧ���¶ȷ�Χ�ǣ�_____��
A 0��C~20��C
B 20��C ~40��C
C 40��C ~60��C
D 60��C ~80��C
II.������Ϊ30g��B���ʼ��뵽50gˮ�У���ֽ����ʣ�����ʵ��������¶ȵĹ�ϵ��ͼ��ʾ�������в�����ˮ���������ش�����:
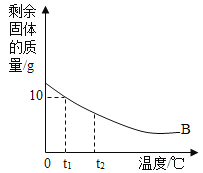
(5)����B���ܽ�����¶ȵ����߶�____________(�����С��) ��
(6) t1��ʱ��B���ܽ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2019��㶫ʡ������������п�һģ��ѧ�Ծ� ���ͣ������
ij��ҵ���ס������������ŷŵķ�ˮ�к���K+��Cu2+�� Fe3+��C1-��OH-��NO3-�������ӣ��׳��������е����֣��ҳ������������֣�������ˮ��ֱ���ŷŶԵ���ˮ�ʴ�������Ӱ�졣ij����ʵ��С���������ˮ����ʵ�ؼ�⣬���ּ׳���ˮ�Լ��ԡ�
��1���׳���ˮ�п϶����е�������_____________,���ܺ��е�������_____________��
��2��������ʵ��С����ʵ�飬����������ˮ���ʵ�������ϣ��ɽ���ˮ�е�ijЩ����ת��Ϊ��������Щ�����ֱ���________________(�ѧʽ),���˺�ķ�ˮ����Ҫ����___________��������(�ѧʽ)��������ķ�ˮ�����ŷű���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2019��㶫ʡ������������п�һģ��ѧ�Ծ� ���ͣ���ѡ��
�Ȼ��(NH4Cl)��ũҵ�����г��õĻ�ѧ���ϡ���֪�Ȼ�淋�ˮ��Һ�����ԣ����й����Ȼ�淋�˵����ȷ����
A.�Ȼ��������
B.�Ȼ����һ�ָ��Ϸ�
C.�Ȼ������ʯ�һ��ʹ�ÿɼ��������ữ������߷�Ч
D.��������Һ�����Ȼ����Һ�н��������ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2019������ʯ��ɽ��ƽ�����п���ģ��ѧ�Ծ� ���ͣ�ʵ����
ʵ���ҳ���������װ����������Ʊ�������ʵ�飮��ش�

��1��д��ͼ�������ٵ����ƣ�______��
��2��ʵ������ȡ������̼���壬�ڼ���ҩƷǰ������е�һ��������_____��ѡ�õ����巢��װ����__������ţ����������Ļ�ѧ��Ӧ����ʽΪ________��
��3��ʵ����������غͶ���������ȡ�����Ļ�ѧ����ʽΪ_______�����ù���������Һ�Ͷ���������ȡ�������������ѡ���巢����������ռ�װ�õĽӿ�����˳����b��__��__��__���ýӿ���ĸ��ʾ����
��4���Ѽ��������ļ���ƿ�����ڵ�����ɫ��̪��Һ��ˮ���У��۲쵽����ƿ���д����ĺ�ɫҺ����룬����������Ϣ�ܽ��������һ������_____���簱���л�������ˮ������_____����ܡ����ܡ�����Eװ�ø��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������2015-2016ѧ����꼶��ѧ�����л�ѧ�Ծ� ���ͣ���ѡ��
ij��Һ���ʿ�����NaOH��HCl��H2SO4��MgCl2��һ�ֻ��֡������Һ�еμ�Ba��OH��2��Һ������������������y�������Ba��OH��2��Һ������x���Ĺ�ϵ����ͼ��ʾ�������й�������ɵ�˵����ȷ����
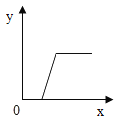
A.HCl��H2SO4һ����
B.H2SO4��MgCl2һ���У�NaOHһ����
C.HCl��MgCl2һ����
D.HClһ���У�NaOH��H2SO4һ���ޣ�MgCl2������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com