
Ζ÷Έω ΗυΨίΗχ≥ωΒΡΉΣΜ·ΙΊœΒΕ‘”ΠΒΡΙΐ≥ΧΖ÷ΈωΟΩΗωΕ‘”ΠΒΡΈ ΧβΘ§Μρ’Ώ÷±Ϋ”Ζ÷ΈωΟΩΗωΈ ΧβΘ§¥”Ηχ≥ωΒΡ–≈œΔ÷–’“Ε‘”ΠΒΡ–≈œΔΘ°
Ϋβ¥π ΫβΘΚ
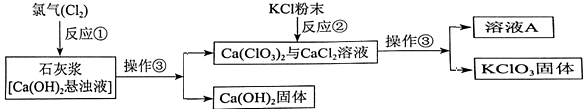
Θ®1Θ©”…”Ύ«β―θΜ·ΗΤΒΡ»ήΫβΕ»ΦΪ–ΓΘ§Υυ“‘ Ι”Ο≥Έ«ε ·Μ“Υ°≤Μ»γ ·Μ“»ι”–ΗϋΕύΒΡ«β―θΜ·ΗΤΫχ»κΖ¥”ΠΘ§Ζ¥”ΠΔΌ÷–”Ο ·Μ“Ϋ§Εχ≤Μ”Ο≥Έ«ε ·Μ“Υ°ΒΡΡΩΒΡ « «β―θΜ·ΗΤΈΔ»ήΘ§ ·Μ“Υ°÷–«β―θΜ·ΗΤΚ§ΝΩΧΪ…ΌΘ§Ζ¥”ΠΚσ…ζ≥…ΈοœύΕ‘Ϋœ…ΌΘ°Μρ ·Μ“Ϋ§÷–«β―θΜ·ΗΤΕύΘ§”–άϊ”Ύ…ζ≥…ΗϋΕύΒΡ…ζ≥…ΈοΘ§
Θ®2Θ©≤ΌΉςΔέ «ΙΧΧεΚΆ“ΚΧεΒΡΖ÷άκΘ§Υυ“‘Ιΐ¬ΥΘΜ
Θ®3Θ©”…”Ύ¬»ΥαΦΊΒΡ»ήΫβΕ»ΥφΈ¬Ε»…ΐΗΏΕχ‘ω¥σΘ§Υυ“‘œ¥Β”KC1O3ΙΧΧε ±Θ§ΈΣΦθ…Ό≤ζΤΖΒΡΥπ ßΘ§œ¥Β”ΦΝΉνΚϔϱΞΚΆΒΡ¬»ΥαΦΊ»ή“ΚΘ§’β―υ¬»ΥαΦΊΒΡΥπ ßΉνΒΆΘ°
Θ®4Θ©”…”ΎΩΣ Φ Ι”ΟΝΥ«β―θΜ·ΗΤΘ§ΉνΚσ”÷…ζ≥…ΝΥ«β―θΜ·ΗΤΘ§Υυ“‘…œ ωΝς≥Χ÷–ΜΊ ’ΚσΩ…“‘‘ΌΉςΈΣ‘≠Νœ Ι”ΟΒΡΈο÷ « CaΘ®OHΘ©2Θ®–¥Μ·―ß ΫΘ©Θ°
Θ®5Θ©¥ΩΕ»ΈΣ98%ΒΡ¬»ΥαΦΊΙΧΧε2.5gΥυΚ§¬»ΥαΦΊΒΡ÷ ΝΩΈΣ98%ΓΝ2.5g=2.45g
…ηάμ¬έ…œΉνΕύΩ…“‘…ζ≥…―θΤχΒΡ÷ ΝΩΈΣx
2KClO3$\frac{\underline{MnO_2}}{Γς}$2KCl+3O2Γϋ
245 96
2.45g x
$\frac{245}{96}$=$\frac{2.45g}{x}$
x=0.96g
Ι ¥πΑΗΈΣΘΚ
Θ®1Θ©«β―θΜ·ΗΤΈΔ»ήΘ§ ·Μ“Υ°÷–«β―θΜ·ΗΤΚ§ΝΩΧΪ…ΌΘ§Ζ¥”ΠΚσ…ζ≥…ΈοœύΕ‘Ϋœ…ΌΘ°Μρ ·Μ“Ϋ§÷–«β―θΜ·ΗΤΕύΘ§”–άϊ”Ύ…ζ≥…ΗϋΕύΒΡ…ζ≥…ΈοΘΜ
Θ®2Θ©Ιΐ¬ΥΘΜ
Θ®3Θ©CΘΜ
Θ®4Θ©CaΘ®OHΘ©2ΘΜ
Θ®5Θ©0.96gΘ°
ΒψΤά ΕΝΆΦΘ§¥”÷–ΜώΒΟΫβ¥πΧβΡΩΥυ–ηΒΡ–≈œΔΘ§Υυ“‘‘ΎΫβ¥πΧβΡΩ ±œ»Ω¥Ϋβ¥πΒΡΈ Χβ « ≤Ο¥Θ§»ΜΚσ¥χΉ≈Έ Χβ»ΞΕΝΗχ≥ωΒΡΆΦΫχΕχ»Ξ―Α’“Ϋβ¥π”–”ΟΒΡ–≈œΔΘ§’β―υΧαΗΏΝΥ–≈œΔΤΥΉΫΒΡ”––ß–‘Θ°Ϋβ¥πΒΡΈ Χβ ΒΦ …œ”κΗ¥‘”ΒΡΉΣΜ·ΆΦœύ±»Θ§Τδ ΒΚήΦρΒΞΚήΜυ¥ΓΘ§Μρ’ΏΩ…“‘ΥΒΉΣΜ·ΆΦΧαΙ©ΒΡ ««ιΨ≥Θ§ΩΦ≤ιΜυ±Ψ÷Σ ΕΘ°



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° |  Ηχ“ΚΧεΦ”»» | BΘ° |  ≤βΕ®Ω’Τχ÷–―θΤχΒΡΚ§ΝΩ | ||
| CΘ° |  ―ι÷ΛCO2ΒΡ–‘÷ | DΘ° |  ’Φ·«βΤχ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | RΘΨCu | BΘ° | RΘΨAg | CΘ° | ZnΘΨR | DΘ° | RΘΨFe |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | 16Γψ C | BΘ° | 20Γψ C | CΘ° | 22Γφ | DΘ° | 30Γφ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΕύ―ΓΧβ
| AΘ° | SΓζSO3 | BΘ° | Na2CO3ΓζNaOH | CΘ° | ΒΑΑΉ÷ ΓζΑ±ΜυΥα | DΘ° | ΒμΖέΓζΨΤΨΪ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | Ba2+ΓΔCu2+ΓΔSO${\;}_{4}^{2-}$ | BΘ° | K+ΓΔBa2+ΓΔNO${\;}_{3}^{-}$ | ||
| CΘ° | Zn2+ΓΔNa+ΓΔCO${\;}_{3}^{2-}$ | DΘ° | Ba2+ΓΔNH${\;}_{4}^{+}$ΓΔOH- |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | Ωσ»ΣΥ° | BΘ° | ≈ΘΡΧ | CΘ° | ΕΙΫ§ | DΘ° | ’τΝσΥ° |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| ―Γœν | Έο÷ | ‘”÷ | ‘ΦΝΓΔΖΫΖ® |
| A | Εΰ―θΜ·ΧΦ | “Μ―θΜ·ΧΦ | Ά®ΙΐΉΤ»»ΒΡ―θΜ·Ά≠ |
| B | ΡΨΧΩΖέ | ΧζΖέ | ”Ο¥≈ΧζΈϋ“ΐ |
| C | NaOH»ή“Κ | Na2CO3 | Φ”»κΙΐΝΩBaΘ®OHΘ©2»ή“ΚΘ§Ιΐ¬Υ |
| D | ―θΜ·Ά≠ | Ά≠Ζέ | ‘ΎΩ’Τχ÷–ΉΤ…’ |
| AΘ° | A | BΘ° | B | CΘ° | C | DΘ° | D |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | ΖΩΈίΉ≈ΜπΘ§Ω…“‘”ΟΥ°ΟπΜπ | |
| BΘ° | ΆΦ ιΙίΉ≈ΜπΘ§”Π Ι”Ο“ΚΧ§Εΰ―θΜ·ΧΦΟπΜπ | |
| CΘ° | “ΡΎΤπΜπΘ§―ΗΥΌ¥ρΩΣΥυ”–Ο≈¥ΑΆ®Ζγ | |
| DΘ° | Φ“”ΟΒγΤςΉ≈ΜπΝΔΦ¥«–ΕœΒγ‘¥ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com