
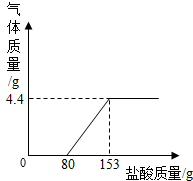
��3�֣�ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ150g����μ���ϡ���������������������������������ϡ����������ϵ����ͼ��ʾ����ʾ:NaOH+HCl=NaCl+H2O��Na2CO3 + 2HCl ="
2NaCl" + H2O + CO2���� ���÷�ˮ��Na2CO3����������������ϡ���������ʵ����������ֱ��Ƕ��٣�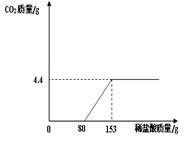
7.1% 10%
��������
����������⣺���ˮ�к�Na2CO3������Ϊx����Na2CO3��Ӧ��ϡ���������ʵ�����Ϊy
Na2CO3 + 2HCl =" 2NaCl" + H2O + CO2��
106 73 44
x y 4.4g
106��44=x��4.4g
x=10.6g
73��44=y��4.4g
y=7.3g
Na2CO3%=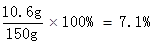
HCl%=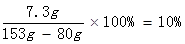
�𣺸÷�ˮ��Na2CO3����������������ϡ���������ʵ����������ֱ���7.1%��10%��
���㣺���ݻ�ѧ����ʽ���㣻���ʵ�����������
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢��
������������=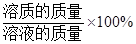 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��ɫ��ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����μ���ϡ���������������������������������ϡ����������ϵ����ͼ��ʾ��
��2012?��ɫ��ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����μ���ϡ���������������������������������ϡ����������ϵ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?�ӱ���һģ��ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����μ���ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ��
��2013?�ӱ���һģ��ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����μ���ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����μ���ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ��
ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����μ���ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
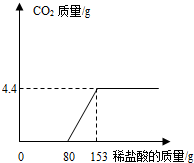
 ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����εμ�ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ��
ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ100g����εμ�ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ150g����μ���ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ����ʾ��NaOH+HCl=NaCl+H2O��Na2CO3+2HCl=2NaCl+H2O+CO2�������÷�ˮ��Na2CO3����������������ϡ���������ʵ����������ֱ��Ƕ��٣�
ij��ֽ���ŷŵķ�ˮ�к���Na2CO3��NaOH��Ϊ�˲ⶨ��ˮ��Na2CO3������������ȡ��ˮ150g����μ���ϡ���������������������������������ϡ����������ϵ��ͼ��ʾ����ʾ��NaOH+HCl=NaCl+H2O��Na2CO3+2HCl=2NaCl+H2O+CO2�������÷�ˮ��Na2CO3����������������ϡ���������ʵ����������ֱ��Ƕ��٣��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com