
| ѳʷ | µŚ1·Ż | µŚ2·Ż | µŚ3·Ż | µŚ4·Ż |
| ȔѳʷµÄÖŹĮæ£Øg£© | 20 | 20 | 20 | 20 |
| Č”ŃĪĖįµÄÖŹĮæ£Øg£© | 50 | 100 | 150 | 200 |
| ²śÉśĘųĢåÖŹĮæ£Øg£© | 2.2 | m | 6.6 | 6.6 |
| 100 |
| x |
| 44 |
| 6.6g |
| 15g |
| 20g |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī | µŚĪå“Ī | µŚĮł“Ī | |
| ¼ÓČėĻ”ĮņĖįµÄÖŹĮæ£Øg£© | 10 | 10 | 10 | 10 | 10 | 10 |
| Ź£Óą¹ĢĢåµÄÖŹĮæ£Øg£© | 9.35 | n | 8.05 | 7.4 | 6.75 | 6.75 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
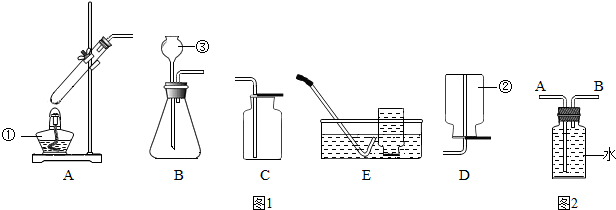
| ×鱚 | Ņ©Ę· | ŹµŃéĻÖĻó |
| ¢Ł | Ģ¼ĖįÄĘ·ŪÄ©ŗĶĻ”ŃĪĖį | ²śÉśĘųÅŻĖŁĀŹŗÜæģ |
| ¢Ś | æéדŹÆ»ŅŹÆŗĶĻ”ĮņĖį | ²śÉśĘųÅŻĖŁĀŹ»ŗĀż²¢Öš½„Ķ£Ö¹ |
| ¢Ū | æéדŹÆ»ŅŹÆŗĶĻ”ŃĪĖį | ²śÉśĘųÅŻĖŁĀŹŹŹÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
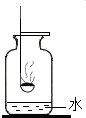 ijĶ¬Ń§¶Ō½Ģ²ÄÖŠĮņŌŚŃõĘųÖŠČ¼ÉÕµÄŹµŃé½ųŠŠĮĖøĽų£®¾ßĢå²Ł×÷ŹĒ£ŗŌŚČ¼ÉÕ³×ÖŠ·ÅÉŁĮæĮņ·Ū£¬µćČ¼ŗóŃøĖŁÉģČė³äĀśŃõĘųĒŅµ×²æÓŠ½Ļ¶ąĮæĖ®µÄ¼ÆĘųĘæÖŠ£ØČēĶ¼ĖłŹ¾£©£®¹Ū²ģµ½ŹµŃéĻÖĻóŗó£¬Į¢¼“½«Č¼ÉÕ³×½žČėĘæÄŚĖ®ÖŠ£®Ēė·ÖĪöŹµŃé¹ż³Ģ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
ijĶ¬Ń§¶Ō½Ģ²ÄÖŠĮņŌŚŃõĘųÖŠČ¼ÉÕµÄŹµŃé½ųŠŠĮĖøĽų£®¾ßĢå²Ł×÷ŹĒ£ŗŌŚČ¼ÉÕ³×ÖŠ·ÅÉŁĮæĮņ·Ū£¬µćČ¼ŗóŃøĖŁÉģČė³äĀśŃõĘųĒŅµ×²æÓŠ½Ļ¶ąĮæĖ®µÄ¼ÆĘųĘæÖŠ£ØČēĶ¼ĖłŹ¾£©£®¹Ū²ģµ½ŹµŃéĻÖĻóŗó£¬Į¢¼“½«Č¼ÉÕ³×½žČėĘæÄŚĖ®ÖŠ£®Ēė·ÖĪöŹµŃé¹ż³Ģ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com