
�����о��ǿ�ѧ�о�����Ҫ����֮һ���������ܽ�����������������е�ʵ�����⡣
(1)��20 g��������Ϊ4%������������Һ��7.3 gϡ�����ϣ�ǡ����ȫ��Ӧ��������Һ��pHΪ7���Լ�������ϡ�������������������
(2)ʵ����Ũ����(������)����������������36%��38%֮��Ϊ�ϸ�ҩƷ��(1)���е�ϡ��������һ�����(�����������2 g)��Ũ����(������)��ˮ���ƶ��ɵġ�ͨ�������жϸ�Ũ�����Ƿ�Ϊ�ϸ�ҩƷ��
�����Զ����о�����Ĵ���Ϊ���⣬������Һ���������������ļ����֪ʶ�㡣����ʱ���ɸ��ݻ�ѧ����ʽ������NaOH��Һ��NaOH����������7.3 gϡ�������������������Ȼ����ϡ��ǰ�������������ֲ��䣬����2 gŨ������������������������ж����Ƿ�ϸ���������£�
�⣺(1)��ϡ���������ʵ�����Ϊx
NaOH + HCl====NaCl + H2O
40 36.5
20 g��4% x
40��36.5=(20 g��4%)��x
x=0.73 g
ϡ�����������������Ϊ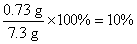
(2)Ũ�����������������Ϊ
36%<36.5%<38%
�ʸ�Ũ����Ϊ�ϸ�ҩƷ(���������ⷨҲ��)
��(1)ϡ�����������������Ϊ10%��
(2)��Ũ�����Ǻϸ�ҩƷ��
�𰸣�(1)10% (2)��Ũ����Ϊ�ϸ�ҩƷ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�ij��г���ѧҵˮƽ���Ի�ѧ�Ծ� ���ͣ�038
�����о��ǿ�ѧ�о�����Ҫ����֮һ���������ܽ���������������е�ʵ�����⣮
(1)��20 g��������Ϊ4��������������Һ��7.3 gϡ�����ϣ�ǡ����ȫ��Ӧ��������Һ��PHΪ7���Լ�������ϡ�������������������
(2)ʵ����Ũ����(������)����������������36����38��֮��Ϊ�ϸ�ҩƷ��(1)���е�ϡ��������һ�����(���������Ϊ2 g)��Ũ����(������)��ˮ���ƶ��ɵģ�ͨ�������жϸ�Ũ�����Ƿ�Ϊ�ϸ�ҩƷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ij� ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com