
| ĪļÖŹ | ±½ | ŃõĘų | ¶žŃõ»ÆĢ¼ | Ė® | X |
| ·“Ó¦Ē°ÖŹĮæ/g | 3.9 | 9.6 | 0 | 0 | 0 |
| ·“Ó¦ŗóÖŹĮæ/g | 0 | 0 | 6.6 | 2.7 | m |
| A£® | ±ķÖŠmµÄÖµĪŖ4.2 | |
| B£® | ĪļÖŹXÓÉĢ¼”¢ŃõŌŖĖŲ×é³É | |
| C£® | ĪļÖŹXÓÉĢ¼”¢ĒāŌŖĖŲ£¬ŃõŌŖĖŲ×é³É | |
| D£® | Éś³É¶žŃõ»ÆĢ¼ŗĶĖ®µÄ·Ö×ÓøöŹż±ČĪŖ2£ŗ1 |
·ÖĪö »Æѧ·“Ó¦×ńŃÖŹĮæŹŲŗć¶ØĀÉ£¬¼“²Ī¼Ó·“Ó¦µÄĪļÖŹµÄÖŹĮæÖ®ŗĶ£¬µČÓŚ·“Ó¦ŗóÉś³ÉµÄĪļÖŹµÄÖŹĮæÖ®ŗĶ£®
½ā“š ½ā£ŗøł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬Éś³ÉXµÄÖŹĮæĪŖ£ŗ3.9g+9.6g-6.6g-2.7g=4.2g£¬
·“Ó¦Ē°ø÷ŌŖĖŲµÄÖŹĮæ£ŗ
3.9g±½ÖŠĢ¼ŌŖĖŲµÄÖŹĮæĪŖ£ŗ3.9g”Į$\frac{72}{78}$”Į100%=3.6g£¬ĒāŌŖĖŲÖŹĮæĪŖ£ŗ3.9g-3.6g=0.3g£¬ŃõĘųÖŠŃõŌŖĖŲµÄÖŹĮæĪŖ9.6g£»
·“Ó¦ŗóø÷ŌŖĖŲµÄÖŹĮæ
6.6g¶žŃõ»ÆĢ¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæĪŖ£ŗ6.6g”Į$\frac{12}{44}$”Į100%=1.8g£¬ŃõŌŖĖŲµÄÖŹĮæĪŖ£ŗ6.6g-1.8g=4.8g£¬
2.7gĖ®ÖŠĒāŌŖĖŲµÄÖŹĮæĪŖ£ŗ2.7g”Į$\frac{2}{18}$”Į100%=0.3g£¬ŃõŌŖĖŲÖŹĮæĪŖ£ŗ2.7g-0.3g=2.4g£¬
¹Ū²ģ·¢ĻÖ£¬±½ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ“óÓŚ¶žŃõ»ÆĢ¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮ棬ŃõĘųÖŠŃõŌŖĖŲµÄÖŹĮæ“óÓŚ¶žŃõ»ÆĢ¼ŗĶĖ®ÖŠµÄŃõŌŖĖŲÖŹĮæŗĶ£¬±½ÖŠĒāŌŖĖŲµÄÖŹĮæµČÓŚĖ®ÖŠĒāŌŖĖŲµÄÖŹĮ棬Ņņ“ĖÅŠ¶ĻXÖŠÓŠĢ¼ŌŖĖŲŗĶŃõŌŖĖŲ£®
¶žŃõ»ÆĢ¼ŗĶĖ®µÄ·Ö×ÓøöŹż±ČĪŖ£ŗ$\frac{6.6g}{44}£ŗ\frac{2.7g}{18}$=1£ŗ1£¬
ÓɼĘĖćæÉÖŖ£¬±ķÖŠmµÄÖµĪŖ4.2£¬Éś³ÉµÄ¶žŃõ»ÆĢ¼ŗĶĖ®µÄ·Ö×ÓøöŹż±ČĪŖ1£ŗ1£¬ĪļÖŹXÓÉĢ¼”¢ŃõŌŖĖŲ×é³É£»
ŅņĪŖ±½ÖŠµÄĒāŌŖĖŲŗĶÉś³ÉµÄĖ®ÖŠµÄĒāŌŖĖŲÖŹĮæĻąµČ£¬ĖłŅŌXÖŠ²»ŗ¬ÓŠĒāŌŖĖŲ£®
¹ŹŃ”£ŗCD£®
µćĘĄ »Æѧ·“Ó¦Ē°ŗó£¬ŌŖĖŲµÄÖÖĄą²»±ä£¬Ō×ÓµÄÖÖĄą”¢×ÜøöŹż²»±ä£¬ÕāŹĒŹéŠ“»Æѧ·½³ĢŹ½ŗĶ½ųŠŠĻą¹Ų·½Ćę¼ĘĖćµÄ»ł“”£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ī¬ÉśĖŲD2ŹōÓŚÓŠ»ś»ÆŗĻĪļ | B£® | Ī¬ÉśĖŲD2ÖŠŗ¬ÓŠ73øöŌ×Ó | ||
| C£® | Ī¬ÉśĖŲD2µÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ396g | D£® | Ī¬ÉśĖŲD2ÖŠĒāŌŖĖŲµÄÖŹĮæ·ÖŹż×ī“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
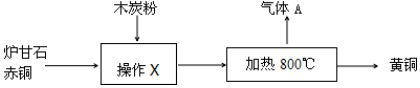
| A£® | ²Ł×÷XµÄĆū³ĘŹĒČܽā | |
| B£® | ¼ÓČČ¹ż³ĢÖŠ·¢ÉśµÄ·“Ó¦Ņ»¶Ø²»ŹĒ»ÆŗĻ·“Ó¦ | |
| C£® | ĘųĢåAæÉÄÜŹĒ»ģŗĻĪļ | |
| D£® | Ļņ»ĘĶÖŠ¼ÓČėĻ”ŃĪĖį£¬»į²śÉśĘųÅŻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ĪļÖŹ | W | X | Y | Z |
| ·“Ó¦Ē°ÖŹĮæ/g | 1.8 | 22 | 34 | 0 |
| ·“Ó¦ŗóÖŹĮæ/g | 1.8 | 40 | Ī“ÖŖ | 16 |
| A£® | ·“Ӧɜ³É40gµÄX | B£® | ”°Ī“ÖŖ”±µÄÖµŹĒ0 | ||
| C£® | WæÉÄÜŹĒ·“Ó¦µÄ“߻ƼĮ | D£® | YŅ»¶ØŹĒ»ÆŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
 A”«F¾łĪŖ³õÖŠ»Æѧ³£¼ūµÄĪļÖŹ£¬ĖūĆĒÖ®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£Ø”°”ś”±±ķŹ¾×Ŗ»Æ¹ŲĻµ£¬ĖłÉę¼°·“Ó¦¾łĪŖ³õÖŠ³£¼ūµÄ»Æѧ·“Ó¦£©£¬ĘäÖŠAĖ׳ĘÉśŹÆ»Ņ£¬CŹĒ×ī³£ÓƵÄČܼĮ£¬CÓėFŌŖĖŲ×é³ÉĻąĶ¬£®
A”«F¾łĪŖ³õÖŠ»Æѧ³£¼ūµÄĪļÖŹ£¬ĖūĆĒÖ®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£Ø”°”ś”±±ķŹ¾×Ŗ»Æ¹ŲĻµ£¬ĖłÉę¼°·“Ó¦¾łĪŖ³õÖŠ³£¼ūµÄ»Æѧ·“Ó¦£©£¬ĘäÖŠAĖ׳ĘÉśŹÆ»Ņ£¬CŹĒ×ī³£ÓƵÄČܼĮ£¬CÓėFŌŖĖŲ×é³ÉĻąĶ¬£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com