
 ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�| XX�� ����̼��� ���������ƣ�35% ������25kg XX��������Ʒ |
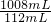 ��0.22g=1.98g
��0.22g=1.98g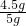 ��100%=90%
��100%=90% ��100%=1.8g
��100%=1.8g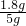 ��100%=36%��35%
��100%=36%��35%

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
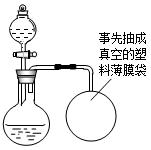 ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�| XX�� ����̼��� ���������ƣ�35% ������25kg XX��������Ʒ�ټ��װ�õ������ԣ� ����Բ����ƿ�м�����Ʒ5.0g�� ��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ �ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL�� ��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������ ��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ����ʲ������ᷴӦ��Ҳ������Ԫ�أ�����ǩ������ͼ��Ϊ�˲ⶨ�京��������С�飮���õ�����ƽ�ⶨ���й�ʵ���������±��� 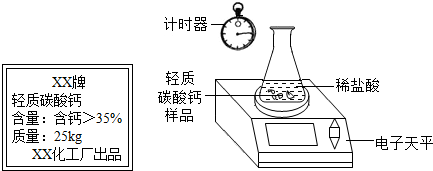
��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�Ͼ������������꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������ ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ����ʲ������ᷴӦ��Ҳ������Ԫ�أ�����ǩ������ͼ��Ϊ�˲ⶨ�京��������С�飮���õ�����ƽ�ⶨ���й�ʵ���������±��� 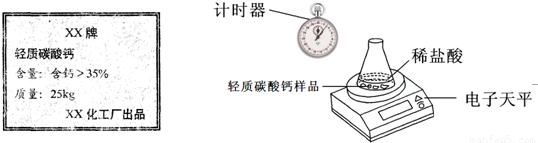
��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2009-2010ѧ�꽭��ʡ�����й�ҵ�����꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������ ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
����Բ����ƿ�м�����Ʒ5.0g�� ��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ �ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL�� ��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������ ��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��  �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |