
 ��
�� 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| [С����] �ټ�ʯ���������ơ��������ƵĹ�����������տ����е�ˮ�����Ͷ�����̼�� ���Ȼ�����Һ�����ԣ�[�������]ʵ�����о��õļ�ʯ����Ʒ�ijɷ���ʲô�� [���в���]���õļ�ʯ����Ʒ�п��ܺ���CaO��NaOH��Na2CO3��Ca��OH��2��CaCO3�ȳɷ֣��û�ѧ����ʽ��ʾ��Ʒ�к���Ca��OH��2��Na2CO3���ܺ��е�ԭ�� CaO+H2O�TCa��OH��2����2NaOH+CO2�TNa2CO3+H2O�ȣ��𰸺������ɣ� CaO+H2O�TCa��OH��2����2NaOH+CO2�TNa2CO3+H2O�ȣ��𰸺������ɣ� ��дһ������[̽������]  �Իش��������⣺ ��1�������ٵ������� ���� ���� ����ҺC��һ����OH- OH- ���ӣ���2����ҺA�м���CaCl2��Һ��������壬˵����Ʒ��һ������ ̼���� ̼���� ����Ӧ�ķ���ʽΪNa2CO3+CaCl2�T2NaCl+CaCO3�� Na2CO3+CaCl2�T2NaCl+CaCO3�� ����3������ҺA�м���CaCl2��Һ��֤��CaCl2��Һ�����ķ����� ȡ��ҺC���Թ��еμ�̼������Һ���а�ɫ�������ɣ�˵��CaCl2��Һ�ѹ��� ȡ��ҺC���Թ��еμ�̼������Һ���а�ɫ�������ɣ�˵��CaCl2��Һ�ѹ��� ��[ʵ�����] ��������ʵ��������̼������ۺ϶���Һ����ɷֵ�̽�������ж���Ʒ�ɷֵķ�����ȷ���� �ڢ� �ڢ� ������ţ�������Ʒ��һ����NaOH ����Ʒ��һ����Na2CO3 ����Ʒ�к�NaOH��CaO�е�һ�ֻ����� [��չ����] Ϊ�ⶨʵ������һƿ���ʵ��ռ���NaOH�ĺ�����ijͬѧȡ�������ռ���Ʒ������һ������ˮ�õ�200g��Һ���ټ���200gϡ���ᣨ��������ֽ��赽���ٷų�����Ϊֹ��������ҺΪ395.6g����������㣺 ��1����Ӧ������CO2������Ϊ 4.4 4.4 g����2������ȡ�ռ���ƷΪ50.0g������Ʒ��NaOH�����������Ƕ��٣���д��������̣� 78.8%�� 78.8%�� ����3����ͬŨ�ȵ����ᣬ�ֱ���δ���ʡ����ֱ��ʡ���ȫ�����ʣ����ʶ���Na2CO3�����ռ���Ʒ��Ӧ����Ҫ�������������ȣ�����Ԫ�������غ�ĽǶȽ�����ԭ���ǣ� �������ƺ�̼���ƶ���ÿ46g��Ԫ������142g�����ƣ�����98g���� �������ƺ�̼���ƶ���ÿ46g��Ԫ������142g�����ƣ�����98g���� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ�  ��ʯ����ʵ���ҳ��õĸ������ͬѧ��Ϊȷ��һƿ���õġ���ʯ�ҡ��������Ѳ��ֱ��ʻ�ȫ�����ʣ���Ʒ�ijɷ֣���������̽���� ��ʯ����ʵ���ҳ��õĸ������ͬѧ��Ϊȷ��һƿ���õġ���ʯ�ҡ��������Ѳ��ֱ��ʻ�ȫ�����ʣ���Ʒ�ijɷ֣���������̽���� ��������⡿ʵ�����о��õļ�ʯ����Ʒ�ijɷ���ʲô�� �����в��롿 ���õļ�ʯ����Ʒ�п��ܺ���CaO��NaOH��Na2CO3��Ca��OH��2��CaCO3�ȳɷ֣��û�ѧ����ʽ��ʾ��Ʒ�к���Ca��OH��2��Na2CO3���ܺ��е�ԭ�� ��̽�����̡� 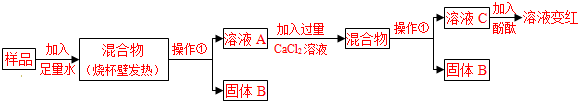 �Իش��������⣺ ��1�������ٵ������� ��2����ҺA�м���CaCl2��Һ��������壬�÷�Ӧ�ķ���ʽΪ ��3������ҺA�м���CaCl2��Һ��֤��CaCl2��Һ�����ķ����� ��ʵ����ۡ� ��������ʵ��������̼������ۺ϶���Һ����ɷֵ�̽�������ж���Ʒ�ɷֵķ�����ȷ���� ����Ʒ��һ����NaOH ����Ʒ��һ����Na2CO3 ����Ʒ�к�NaOH��CaO�е�һ�ֻ����� ����չ���졿 ��1��Ϊ�ⶨʵ������һƿ���ʵ��ռ���NaOH�ĺ�����ijͬѧȡ50.0g�ռ���Ʒ������һ������ˮ�еõ�200g��Һ���ټ���200gϡ���ᣨ��������ֽ��赽���ٷų�����Ϊֹ��������ҺΪ395.6g�������������Ʒ��NaOH�����������Ƕ��٣���д��������̣� ��2����ͬŨ�ȵ����ᣬ�ֱ���δ���ʡ����ֱ��ʡ���ȫ�����ʣ����ʶ���Na2CO3�����ռ���Ʒ��Ӧ����Ҫ�������������ȣ�����Ԫ�������غ�ĽǶȽ�����ԭ���ǣ� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� ��ʯ���������ƺ��������ƵĹ��������ʵ���ҳ��õĸ�����������Ϣ����ͼ��ʾ��ͬѧ��Ϊȷ��һƿ���õġ���ʯ�ҡ��������Ѳ��ֱ��ʻ�ȫ�����ʣ���Ʒ�ijɷ֣���������̽����
[�������]ʵ�����о��õļ�ʯ����Ʒ�ijɷ���ʲô�� [���в���] ���õļ�ʯ����Ʒ�п��ܺ���CaO��NaOH��Na2CO3��Ca��OH��2��CaCO3�ȳɷ֡��û�ѧ����ʽ��ʾ��Ʒ�к���Ca��OH��2��Na2CO3���ܺ��е�ԭ�� ��дһ���� [̽������]
�Իش��������⣺ ��1�������ٵ������� ����ҺC��һ���� ���ӡ� ��2����ҺA�м���CaCl2��Һ��������壬˵����Ʒ��һ������ ����Ӧ�ķ���ʽΪ �� ��3������ҺA�м���CaCl2��Һ��֤��CaCl2��Һ�����ķ����� �� [ʵ�����] ��������ʵ��������̼������ۺ϶���Һ����ɷֵ�̽�������ж���Ʒ�ɷֵķ�����ȷ���� ������ţ� ����Ʒ��һ����NaOH ����Ʒ��һ����Na2CO3 ����Ʒ�к�NaOH��CaO�е�һ�ֻ����� [��չ����] Ϊ�ⶨʵ������һƿ���ʵ��ռ���NaOH�ĺ�����ijͬѧȡ�������ռ���Ʒ������һ������ˮ�õ�200g��Һ���ټ���200gϡ���ᣨ��������ֽ��赽���ٷų�����Ϊֹ��������ҺΪ395.6g����������㣺 ��1����Ӧ������CO2������Ϊ g�� ��2������ȡ�ռ���ƷΪ50.0g������Ʒ��NaOH�����������Ƕ��٣���д��������̣�
��3����ͬŨ�ȵ����ᣬ�ֱ���δ���ʡ����ֱ��ʡ���ȫ�����ʣ����ʶ���Na2CO3�����ռ���Ʒ��Ӧ����Ҫ�������������ȡ�����Ԫ�������غ�ĽǶȽ�����ԭ���ǣ� �� ��������[���в���]�����������Ƶķ���ʽΪCaO+H2O�TCa��OH��2 ����̼���Ƶķ���ʽΪ2NaOH+CO2�TNa2CO3+H2O [̽������] ��1������Һ����IJ���Ϊ���ˣ���̪����ˣ�˵����Һ�Լ��ԣ�����Һ��һ������OH�� ��2�������Ȼ��Ʒ�Ӧ����̼���ƣ��䷴Ӧ����ʽΪNa2CO3+CaCl2�T2NaCl+CaCO3�� ��3������ȡ��ҺC���Թ��еμ�̼������Һ���а�ɫ�������ɣ���˵��CaCl2��Һ�ѹ��� [ʵ�����]��Ϊ��ˮ���ձ��ڱ���˵����Ʒ�к����������ƻ����������ƣ������Ƕ��߶��У������Ȼ������ɰ�ɫ����˵����Ʒ��һ������̼���ƣ��ۺ����Ϸ�������ѡ�ڢ� [��չ����] ��1�����������غ㶨�ɿ������Ӧ������CO2������Ϊ200g+200g-395.6g=4.4g ��2�����ݶ�����̼������Ϊ4.4�˾Ϳ��Լ����ˣ����ݻ�ѧ��Ӧ����ʽ���㣬���ɵó���Ʒ��̼���Ƶ��������̶��ó�ԭ��Ʒ���������Ƶ����������岽����𰸡� ��3���������ƺ�̼���ƶ���ÿ46g��Ԫ������142g�����ƣ�����98g����
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012�꽭��ʡ�Ͼ�����ˮ���п���ѧ��ģ�Ծ��������棩 ���ͣ������ ��ʯ���������ƺ��������ƵĹ��������ʵ���ҳ��õĸ�����������Ϣ���±���ʾ��ͬѧ��Ϊȷ��һƿ���õġ���ʯ�ҡ��������Ѳ��ֱ��ʻ�ȫ�����ʣ���Ʒ�ijɷ֣���������̽����
[���в���]���õļ�ʯ����Ʒ�п��ܺ���CaO��NaOH��Na2CO3��Ca��OH��2��CaCO3�ȳɷ֣��û�ѧ����ʽ��ʾ��Ʒ�к���Ca��OH��2��Na2CO3���ܺ��е�ԭ��______��дһ������ [̽������]  �Իش��������⣺ ��1�������ٵ�������______����ҺC��һ����______���ӣ� ��2����ҺA�м���CaCl2��Һ��������壬˵����Ʒ��һ������______����Ӧ�ķ���ʽΪ______�� ��3������ҺA�м���CaCl2��Һ��֤��CaCl2��Һ�����ķ�����______�� [ʵ�����] ��������ʵ��������̼������ۺ϶���Һ����ɷֵ�̽�������ж���Ʒ�ɷֵķ�����ȷ����______������ţ��� ����Ʒ��һ����NaOH ����Ʒ��һ����Na2CO3 ����Ʒ�к�NaOH��CaO�е�һ�ֻ����� [��չ����] Ϊ�ⶨʵ������һƿ���ʵ��ռ���NaOH�ĺ�����ijͬѧȡ�������ռ���Ʒ������һ������ˮ�õ�200g��Һ���ټ���200gϡ���ᣨ��������ֽ��赽���ٷų�����Ϊֹ��������ҺΪ395.6g����������㣺 ��1����Ӧ������CO2������Ϊ______ g�� ��2������ȡ�ռ���ƷΪ50.0g������Ʒ��NaOH�����������Ƕ��٣���д��������̣�______�� ��3����ͬŨ�ȵ����ᣬ�ֱ���δ���ʡ����ֱ��ʡ���ȫ�����ʣ����ʶ���Na2CO3�����ռ���Ʒ��Ӧ����Ҫ�������������ȣ�����Ԫ�������غ�ĽǶȽ�����ԭ���ǣ�______�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��Ƚ���ʡ�Ͼ�����ˮ�صڶ�ѧ�ڵڶ��ε��в��Ի�ѧ�Ծ��������棩 ���ͣ������� ��ʯ���������ƺ��������ƵĹ��������ʵ���ҳ��õĸ�����������Ϣ����ͼ��ʾ��ͬѧ��Ϊȷ��һƿ���õġ���ʯ�ҡ��������Ѳ��ֱ��ʻ�ȫ�����ʣ���Ʒ�ijɷ֣���������̽����
[�������]ʵ�����о��õļ�ʯ����Ʒ�ijɷ���ʲô�� [���в���] ���õļ�ʯ����Ʒ�п��ܺ���CaO��NaOH��Na2CO3��Ca��OH��2��CaCO3�ȳɷ֡��û�ѧ����ʽ��ʾ��Ʒ�к���Ca��OH��2��Na2CO3���ܺ��е�ԭ�� ��дһ���� [̽������]
�Իش��������⣺ ��1�������ٵ������� ����ҺC��һ���� ���ӡ� ��2����ҺA�м���CaCl2��Һ��������壬˵����Ʒ��һ������ ����Ӧ�ķ���ʽΪ �� ��3������ҺA�м���CaCl2��Һ��֤��CaCl2��Һ�����ķ����� �� [ʵ�����] ��������ʵ��������̼������ۺ϶���Һ����ɷֵ�̽�������ж���Ʒ�ɷֵķ�����ȷ���� ������ţ� ����Ʒ��һ����NaOH ����Ʒ��һ����Na2CO3 ����Ʒ�к�NaOH��CaO�е�һ�ֻ����� [��չ����] Ϊ�ⶨʵ������һƿ���ʵ��ռ���NaOH�ĺ�����ijͬѧȡ�������ռ���Ʒ������һ������ˮ�õ�200g��Һ���ټ���200gϡ���ᣨ��������ֽ��赽���ٷų�����Ϊֹ��������ҺΪ395.6g����������㣺 ��1����Ӧ������CO2������Ϊ g�� ��2������ȡ�ռ���ƷΪ50.0g������Ʒ��NaOH�����������Ƕ��٣���д��������̣�
��3����ͬŨ�ȵ����ᣬ�ֱ���δ���ʡ����ֱ��ʡ���ȫ�����ʣ����ʶ���Na2CO3�����ռ���Ʒ��Ӧ����Ҫ�������������ȡ�����Ԫ�������غ�ĽǶȽ�����ԭ���ǣ� �� ��������[���в���]�����������Ƶķ���ʽΪCaO+H2O�TCa��OH��2 ����̼���Ƶķ���ʽΪ2NaOH+CO2�TNa2CO3+H2O [̽������] ��1������Һ����IJ���Ϊ���ˣ���̪����ˣ�˵����Һ�Լ��ԣ�����Һ��һ������OH�� ��2�������Ȼ��Ʒ�Ӧ����̼���ƣ��䷴Ӧ����ʽΪNa2CO3+CaCl2�T2NaCl+CaCO3�� ��3������ȡ��ҺC���Թ��еμ�̼������Һ���а�ɫ�������ɣ���˵��CaCl2��Һ�ѹ��� [ʵ�����]��Ϊ��ˮ���ձ��ڱ���˵����Ʒ�к����������ƻ����������ƣ������Ƕ��߶��У������Ȼ������ɰ�ɫ����˵����Ʒ��һ������̼���ƣ��ۺ����Ϸ�������ѡ�ڢ� [��չ����] ��1�����������غ㶨�ɿ������Ӧ������CO2������Ϊ200g+200g-395.6g=4.4g ��2�����ݶ�����̼������Ϊ4.4�˾Ϳ��Լ����ˣ����ݻ�ѧ��Ӧ����ʽ���㣬���ɵó���Ʒ��̼���Ƶ��������̶��ó�ԭ��Ʒ���������Ƶ����������岽����𰸡� ��3���������ƺ�̼���ƶ���ÿ46g��Ԫ������142g�����ƣ�����98g����
�鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |