
| ����ϡ��������� | ��ַ�Ӧ��ʣ��������� |
| ��һ�μ���5mL | 1.443g |
| �ڶ��μ���5mL | 0.687 |
| �������5mL | 0.40 |
| ���Ĵμ���5mL | 0.40 |
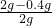 ��100%=80%
��100%=80% CaO+CO2��
CaO+CO2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ϡ��������� | ��ַ�Ӧ��ʣ��������� |
| ��һ�μ���5mL | 1.443g |
| �ڶ��μ���5mL | 0.687 |
| �������5mL | 0.40 |
| ���Ĵμ���5mL | 0.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijУ�о���ѧϰС��Ը���ij���ں�ˮ�ʵ�״��������صĵ����о���
ijУ�о���ѧϰС��Ը���ij���ں�ˮ�ʵ�״��������صĵ����о���| ͨ�� |
| ͨ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2010?���ݣ�ijУ�о���ѧϰС��Բ��÷ֽ����������Һ��ȡ����������ʵ��̽����
��2010?���ݣ�ijУ�о���ѧϰС��Բ��÷ֽ����������Һ��ȡ����������ʵ��̽����| ʵ��� | ���Թ��м���5mL 5%�Ĺ���������Һ |
| ʵ��� | ���Թ��м���5mL 5%�Ĺ���������Һ�������� |
| ʵ��� | ���Թ��м���5mL 5%�Ĺ���������Һ������������MnO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
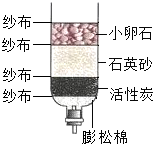 ijУ�о���ѧϰС��Ժ���ij��ˮ�ʵ�״��������صĵ����о���
ijУ�о���ѧϰС��Ժ���ij��ˮ�ʵ�״��������صĵ����о����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ�о���ѧϰС��Ը���ij���ں�ˮ�ʵ�״��������صĵ����о���
ijУ�о���ѧϰС��Ը���ij���ں�ˮ�ʵ�״��������صĵ����о����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com