
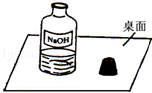
| ʵ�鲽�� | Ԥ�������� |
| | |
| | |
| ʵ�鲽�� | Ԥ�������� |
| ȡ��������Һ���Թܣ���������BaCl2��Һ | ������ɫ��������˵����Һ�к���Na2CO3 |
| ���ú�������Թ��У��μ�������̪��Һ | ��Һ��죬��˵����Һ�к���NaOH |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
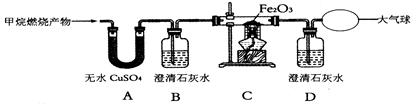
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
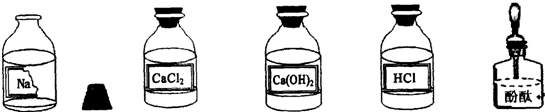
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| MnO2(g) | 0��1 | 0��2 | 0��3 | 0��4 | 0��5 | 0��6 | 0��7 | 0��8 | 0��9 | 1��0 |
| �������� | | | | | | | | | | |
�鿴�𰸺ͽ���>>
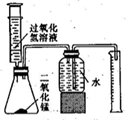
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
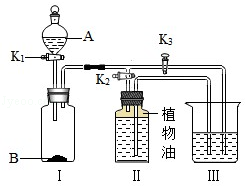
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
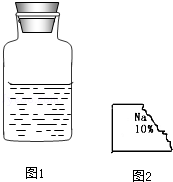
| ���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
| �ܽ��g | 36 | 109 | 215 | 9.6 |
| ʵ�鲽�� | ʵ����������� |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


| ���� | ʵ����� | ʵ������ |
| | ����ʹ�ִ���ҩ�ﵹ��һ�ձ��У��۲� | ҩ��Ϊ��ɫ�����ĩ |
| | ��һ���δ������ձ��еĺ�ɫ��ĩ��ֽӴ���ȡ���������ڴ����ϵĺ�ɫ��ĩ����ȼ�ճ��У��ھƾ����ϵ�ȼ������ʢ�������ļ���ƿ�У��۲� | ����ȼ�գ� |
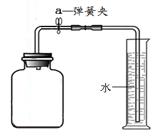
| ���� | ʵ����� | ��Ҫʵ������ | ʵ����ۼ����� |
| | ���Ӻ�װ������ͼ��ʾ��Ȼ�� | | װ�õ����������� |
| | ��������ҩƷ��Ѹ�ٽ��������ϣ�������� | | ���뼯��ƿ��ˮ�������Ϊ�����������ĺ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
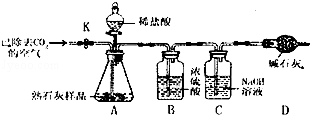
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | ʵ����� | ʵ������ |
| �� | ȡ�����Թ��У�����������ˮ���� | ��Ʒ��ʧ���õ���ɫ������Һ |
| �� | �����������Һ�еμ���ɫ��̪��Һ | ��Һ��� |
| �� | �����������Һ�еμ�ϡ���������� | ������ɫ����ζ���壬��Һ��ɫ |
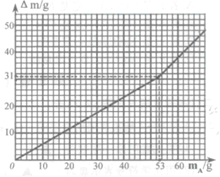
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com