

 CaO+CO2�� CO2 ʳƷ�������
CaO+CO2�� CO2 ʳƷ�������  CaO+CO2�����ù����п�ѭ��ʹ�õ�XΪCO2�����и���ƷY�������ƣ���;�ǣ�ʳƷ�������
CaO+CO2�����ù����п�ѭ��ʹ�õ�XΪCO2�����и���ƷY�������ƣ���;�ǣ�ʳƷ�������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ʯ | B���ϳ��� | C����ͨˮ�� | D��Ӳ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ʵ �� �� �� | �� �� | �� �� |
| ��1���ֱ�������������ۺ���������۷����Թ��У�������ˮ������һ��ʱ��μ� �� | ��������۵��ϲ���Һ��죬��������۵���Һû�б�ɫ | ����ٳ��� |
| ��2���ֱ�������������ۺ���������۷����Թ��У�������ˮ�����ˣ� �����ȡ� | ��������۵���Һ�л�ɫ���֣��ֲ���ڣ���������۵���Һû���������� | ���� ���� |
| | ��������� | ��������� |
| ����۵����� | 100g | 100g |
| ������������� | 460.0g | 500g |
| �ձ����������ʵ������� | 520.0g | 558.2g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
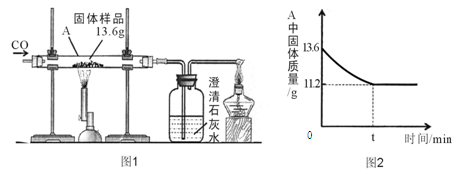
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��7��4�� | B��10�� | C��8�� | D��11��1�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ʵ����� | 1 | 2 | 3 | 4 |
| ϡ�����������g�� | 5 | 5 | 5 | 5 |
| ��Ӧ���ձ������ʵ����� | 15 | 19.56 | 24.12 | 29.12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com