
| ��������������ص������� |
|
|
|
|
|
|
|
|
| ||||||||||||||||||
| ����1L���������ʱ�䣨s�� | 124 | 79 | 50 | 54 | 75 | 93 | 106 | 153 | 240 |
| �������� |
| ���� |
| �������� |
| ���� |


 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鷽�� | ʵ����� | ʵ������ | ʵ����� |
| С�Ƶķ��� | ȡ������ɫ�������Թ��У������м���ϡ���� | �����ܼ�����ɫ��ð�� | ��3�� |
| �ҵķ��� | ��4�� | ��5�� | ��ɫ������Ca��OH��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ɱ� ʯ�� ���� |
| B���� ���ʯ ��Ȫˮ |
| C���ƾ� ��ʯ�� ú |
| D������������ �� ��ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ʵ����� |
| B��Ԫ�ص����� |
| C��ԭ�ӵ���Ŀ |
| D����Ӧǰ�����ʵ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
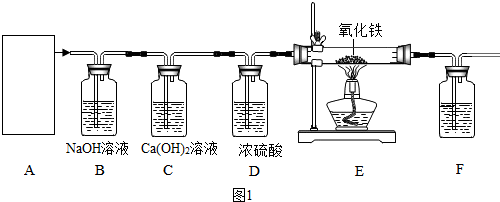
| 400��-500�� |
| 500��-600�� |
| 700��-800�� |
| ���������� | �����ܼ����й�������� | װ��F���������ʵ������� | |
| ��Ӧǰ | 28.20g | 33.00g | 300.0g |
| ��Ӧ�� | 32.84g | 300.4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ˮ����������������ɵ� |
| B��ˮ��һ�������� |
| C��ˮ�����к����⡢������ԭ�� |
| D��ˮ�����к���������ԭ�ӡ�һ����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com