
(8·Ö)A”ŖFŗĶX¶¼ŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ£¬ĘäÖŠA”¢CŹĒĪŽÉ«ĘųĢ壬BĪŖŗģÉ«·ŪÄ©£¬ĖüĆĒµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·ÖÉś³ÉĪļŅŃĀŌČ„£©£ŗ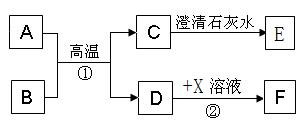
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ŗ¬DµÄÉś²ś”¢Éś»īÓĆĘ·øÆŹ“µÄ¹ż³Ģ£¬Źµ¼ŹÉĻŹĒDÓėæÕĘųÖŠµÄ
·¢Éś»Æѧ·“Ó¦µÄ¹ż³Ģ”£
£Ø3£©Š“³öCÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ӣ
£Ø4£©ČōFĪŖĘųĢåµ„ÖŹ£¬ĒėŠ“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½
øĆ·“Ó¦ŹōÓŚ £ØĢīŠ“»ł±¾·“Ó¦ĄąŠĶ£©·“Ó¦

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
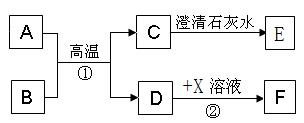
(8·Ö)A”ŖFŗĶX¶¼ŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ£¬ĘäÖŠA”¢CŹĒĪŽÉ«ĘųĢ壬BĪŖŗģÉ«·ŪÄ©£¬ĖüĆĒµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·ÖÉś³ÉĪļŅŃĀŌČ„£©£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ŗ¬DµÄÉś²ś”¢Éś»īÓĆĘ·øÆŹ“µÄ¹ż³Ģ£¬Źµ¼ŹÉĻŹĒDÓėæÕĘųÖŠµÄ
·¢Éś»Æѧ·“Ó¦µÄ¹ż³Ģ”£
£Ø3£©Š“³öCÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ӣ
£Ø4£©ČōFĪŖĘųĢåµ„ÖŹ£¬ĒėŠ“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½
øĆ·“Ó¦ŹōÓŚ £ØĢīŠ“»ł±¾·“Ó¦ĄąŠĶ£©·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ğĞĻÄŅų“ØŹŠ¾ÅÄź¼¶ÉĻѧʌʌĩ¼ģ²ā»Æѧ¾ķ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
(8·Ö)A”ŖFŗĶX¶¼ŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ£¬ĘäÖŠA”¢CŹĒĪŽÉ«ĘųĢ壬BĪŖŗģÉ«·ŪÄ©£¬ĖüĆĒµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·ÖÉś³ÉĪļŅŃĀŌČ„£©£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ŗ¬DµÄÉś²ś”¢Éś»īÓĆĘ·øÆŹ“µÄ¹ż³Ģ£¬Źµ¼ŹÉĻŹĒDÓėæÕĘųÖŠµÄ
·¢Éś»Æѧ·“Ó¦µÄ¹ż³Ģ”£
£Ø3£©Š“³öCÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ӣ
£Ø4£©ČōFĪŖĘųĢåµ„ÖŹ£¬ĒėŠ“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½
øĆ·“Ó¦ŹōÓŚ £ØĢīŠ“»ł±¾·“Ó¦ĄąŠĶ£©·“Ó¦
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com