
��Һ����ɺ������dz��л�ѧ�е���Ҫ֪ʶ���������������й㷺��Ӧ�á�
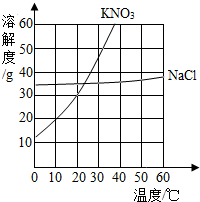
��1����ͼΪ�Ȼ��ơ��������ˮ�е��ܽ�����ߡ�
�Ȼ�����Һ�е��ܼ��� ��20��ʱ������ص��ܽ��ԼΪ g��30��ʱ������ص��ܽ�� �Ȼ��Ƶ��ܽ�ȡ����� �����ڡ�����С�ڡ����ڡ���
��2��ijѧУ��ѧ��ȤС���ò������Ȼ��ƹ���(���е�����CaO)������һ����������������NaCl��Һ��ʵ�������ͼ���£�
����ҺA�е�����Ϊ ��
�ڲ���II�������� ��
�۹���ϡ������뵽��ҺB����������Ӧ�Ļ�ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 29����Һ����ɺ������dz��л�ѧ�е���Ҫ֪ʶ���������������й㷺��Ӧ�ã�
29����Һ����ɺ������dz��л�ѧ�е���Ҫ֪ʶ���������������й㷺��Ӧ�ã�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡɽ�����������ѧ�ڵڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��Һ����ɺ������dz��л�ѧ�е���Ҫ֪ʶ���������������й㷺��Ӧ�á�

��1����ͼΪ�Ȼ��ơ��������ˮ�е��ܽ�����ߡ�
�Ȼ�����Һ�е��ܼ��� ��20��ʱ������ص��ܽ��ԼΪ g��30��ʱ������ص��ܽ�� �Ȼ��Ƶ��ܽ�ȡ����� �����ڡ�����С�ڡ����ڡ���
��2��ijѧУ��ѧ��ȤС���ò������Ȼ��ƹ���(���е�����CaO)������һ����������������NaCl��Һ��ʵ�������ͼ���£�

����ҺA�е�����Ϊ ��
�ڲ���II�������� ��
�۹���ϡ������뵽��ҺB����������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com