


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������������һģ��ѧ�Ծ��������棩 ���ͣ������
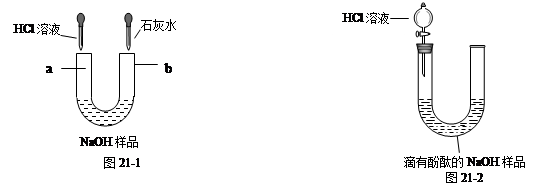
��6�֣�����һƿ���õı�����������Ϊ10%��NaOH��Һ��Ʒ��Ϊ̽������ʣ�NaOH�Ϳ����е�CO2��Ӧ����Na2CO3����������⣬���á��ι��������ͼ��ʾ��װ�ý���ʵ�顣
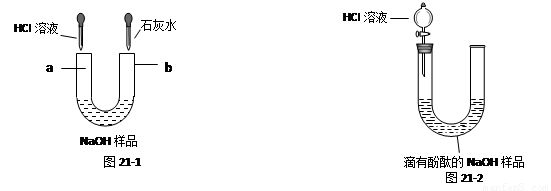
��1����ͼ46-1��ʾ��������Һ���ʣ��ֱ��ڡ��ιܵ����˵���HCl��Һ�ͳ����ʯ��ˮʱ���۲쵽������a�� ��b�� ��
��2����ͼ46-2��ʾ��ȡ��NaOH��Ʒ��Һ20g�ڡ��ι��У��������м��������ķ�̪��Һ��ͨ����Һ©������ι��е���HCl��Һ��������20gHCl��Һʱ��ǡ�÷�Ӧ��ȫ��
�ٵ���ϡ��������У����ι��е���Һ��ɫ�仯Ϊ ��
��ǡ����ȫ��Ӧʱ����������0.22g����ͨ������ó�20gNaOH��Ʒ��Һ������Na2CO3��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�������������п���ѧһģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com