
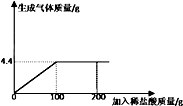
 ��ѧ��ȤС���ͬѧ�������ʵ�����뼼��Աһ�����ʯ��ʯ��Ʒ��̼��ƣ���ȡ12g��Ʒ���μ�7.3%��ϡ���ᣨ��Ʒ�е����ʲ���ϡ���ᷴӦ�����μ�ϡ���������������������ϵ����ͼ����
��ѧ��ȤС���ͬѧ�������ʵ�����뼼��Աһ�����ʯ��ʯ��Ʒ��̼��ƣ���ȡ12g��Ʒ���μ�7.3%��ϡ���ᣨ��Ʒ�е����ʲ���ϡ���ᷴӦ�����μ�ϡ���������������������ϵ����ͼ�������� ̼�����ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ɵμ�ϡ���������������������ϵͼ��ǡ����ȫ��Ӧʱ����ϡ���������Ϊ4.4g����ʱ����ϡ���������Ϊ100g���ݴ��ɷ�Ӧ�Ļ�ѧ����ʽ��ʽ������μӷ�Ӧ��̼��Ƶ����������������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��� �⣺��1����ͼʾ��֪����̼�����ϡ����ǡ����ȫ��Ӧʱ������7.3%ϡ�����������100g��
��2����Ҫ��ˮ������Ϊx��������Һϡ��ǰ�����ʵ��������䣬
��250g��7.3%=36.5%x x=50g��
��3����μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
$\frac{100}{44}=\frac{x}{4.4g}$ x=10g
ʯ��ʯ��Ʒ��̼��Ƶ�����������$\frac{10g}{12g}$��100%��83.3%��
�𣺣�1��100����2��50����3��ʯ��ʯ��Ʒ��̼��Ƶ�����������83.3%��
���� �����ѶȲ������ո��ݻ�ѧ����ʽ�ļ��㼴����ȷ����⣬ϸ�µط���ͼ����Ϣ��ȷ�����ɶ�����̼�������������ȷ������ǰ��ؼ���


 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ���� | �����Լ� | ���ӷ��� | |
| A | H2O | MnO2 | ����̿ | ���������� |
| B | ���� | ͭ�� | ���� | �����������ᣬ��ַ�Ӧ����ˣ�ϴ�ӣ����� |
| C | CO2 | CO | O2 | ��ȼ |
| D | NaCl | Na2CO3 | ���� | �������������ٲ������ݣ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϡ��������⣺Fe2O3+6HCl��2FeCl2+3H2O | |
| B�� | ��ҵ��������������̼���ƣ�Na2CO3+Ca��OH��2��CaCO3��+2NaOH | |
| C�� | �õ��ˮ�ķ����ⶨˮ����ɣ�2H2O��2H2��+O2�� | |
| D�� | �ö�����̼��̼�����ϣ�CO2+H2O��H2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl | B�� | H2SO4 | C�� | NaCl | D�� | H2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ɱ����� | B�� | ����ե֭ | C�� | ˮש��ˮ | D�� | ֲ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KNO3 | B�� | Ca��OH��2 | C�� | BaSO4 | D�� | CaCO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com