�⣺��1���������������--��ѹ�����������˲���־������õĵ����ԣ�
���B��
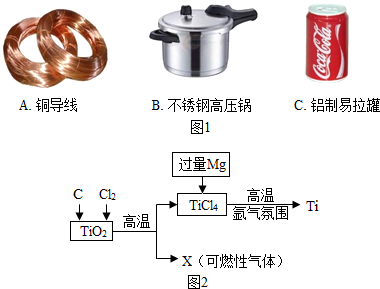
��2����TiO
2��C��ϣ��ڸ��������·�Ӧ����ͨ��Cl
2��������TiCl
4�⣬���ܹ�����һ����̼���壮
���һ����̼��
��Mg��TiCl
4�ڸ��������·�Ӧ��������Ti��MgCl
2����ѧ����ʽΪ��2Mg+TiCl
4
Ti+2MgCl
2��
���2Mg+TiCl
4
Ti+2MgCl
2��
����Ļ�ѧ���ʺܲ����ã�����������ȡ�����ѵı�������
���������������ֹ����������Ӧ��
��3���⣺��������������Ϊx��
ͭ���������ᷴӦ���ܲ���������ֻ��п����ϡ����ķ�Ӧ��
Zn+H
2SO
4�TZnSO
4+H
2����
65 2
13g x
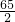
=
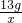
��
x=0.4g��
�𣺿���������0.4g��
��������1�������������õĵ����ԡ������ԡ���չ�ԣ�
��2�����������غ㶨�ɺ���ط������Ϣ�����ж����ʵ����ƣ����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��ѧ����ʽ��
��3�����ݻ�ѧ����ʽ���Լ�������������������
������������Ҫ�������ʵ����ʺ���;����ѧ����ʽ����д�����ݻ�ѧ����ʽ����ȷ����֪ʶ�����ݻ�ѧ����ʽ����Ƚϼ�����ʱֻҪע��淶�Ա��˳�����

 �������������������й㷺��Ӧ�ã�
�������������������й㷺��Ӧ�ã� Ti+2MgCl2��
Ti+2MgCl2�� Ti+2MgCl2��
Ti+2MgCl2��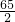 =
=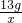 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�

 ��2013?��������ģ���������������������й㷺��Ӧ�ã�
��2013?��������ģ���������������������й㷺��Ӧ�ã� ��2013?��������ģ���������������������й㷺��Ӧ�ã�
��2013?��������ģ���������������������й㷺��Ӧ�ã�