
 ���x=20g�����ݣ�
���x=20g�����ݣ�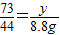 ���y=14.6g��
���y=14.6g�� ×100%=25g��
×100%=25g��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ʯ��ʯ��Դ�ḻ����Ϊ���з�չ���Ĺ�ҵ�ṩ�˵�����������������ijУ���꼶��ѧ�о���ѧϰС���ͬѧ���ڿ����̽����вɼ��˵���һ��ɽ�ϵ�ʯ��ʯ��Ʒ����ͨ��ʵ��ⶨʯ��ʯ��Ʒ��̼��Ƶĺ���������ȡ100gʯ��ʯ��Ʒ�����ʲ������ˮ��Ӧ�������ձ��в�����300gϡ���ᣬǡ����ȫ��Ӧ���Ѳ����Ķ�����̼������������ʯ��ˮ���գ�ͬʱ����3�������ձ������ص�������������±���
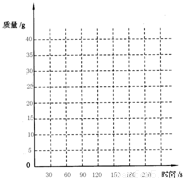
����ʯ��ʯ��Դ�ḻ����Ϊ���з�չ���Ĺ�ҵ�ṩ�˵�����������������ijУ���꼶��ѧ�о���ѧϰС���ͬѧ���ڿ����̽����вɼ��˵���һ��ɽ�ϵ�ʯ��ʯ��Ʒ����ͨ��ʵ��ⶨʯ��ʯ��Ʒ��̼��Ƶĺ���������ȡ100gʯ��ʯ��Ʒ�����ʲ������ˮ��Ӧ�������ձ��в�����300gϡ���ᣬǡ����ȫ��Ӧ���Ѳ����Ķ�����̼������������ʯ��ˮ���գ�ͬʱ����3�������ձ������ص�������������±���| ʱ��/s | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
| ����/g | 0 | 15 | 25 | 30 | 33 | 33 | 33 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2005-2006ѧ�갲��ʡ�����оӳ������꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
| ʱ��/s | 30 | 60 | 90 | 120 | 150 | 180 | |
| ����/g | 15 | 25 | 30 | 33 | 33 | 33 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com