



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
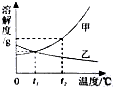 ��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�
��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�������о��꼶3���¿���ѧ�Ծ��������棩 ���ͣ������
ʵ�����ṩ������ƽ����Ͳ���ձ������������ƾ��Ƶ�������С��ͬѧ�ù����Ȼ�������10�����Ȼ�����Һ��С��ͬѧ�ù���������������10��������������Һ��С��ͬѧ��Ũ��������10����ϡ���ᡣ
��1��С��ͬѧ���ƹ������ò�����������Ϊ��???????????????????? ��
��2��С������Ͳ��ȡˮʱ���Ӷ���������������������Һ������������?????? 10��(��������������С��������������)��
��3��С��ͬѧ����10����ϡ����IJ����У���ϡ����������װƿ������ǩ����ȡŨ�����ˮ������ȷ��˳��Ϊ?????? (�����)��
��4�����������У���λͬѧ������Ҫ��������?????? (����ţ���ͬ)С����С����Ҫ��С������Ҫ��������????????????? ��
��5�����������˸����Ƶ���ƿ��Һ���ϱ�ǩ�����δ����ǩ����ƿ��Һ���м��������
������ѡ�����Խ���ƿ��Һ����������Լ�????????????? ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com