13���±�Ϊ�ҹ�1998�껯ʯȼ�ϴ����������

��1������ݱ����������ݣ���д����
20�꣬63�꣬92��
��2�����ݱ������ǵ���ʾ�ǣ�����ţ�
�ڢۢ�
�ٻ�ʯȼ���̲���������
�ڻ�ʯȼ���Dz�����������Դ�����ٱ��ľ���Σ��
�ۻ�ʯȼ��Ӧ�������ɡ��ۺ����á���Լʹ��
������Ŀǰ�����ĵ�������Ҫ���Ի�ʯȼ��
��3��Ϊ��Լȼ�ϣ���ȡ�ľ����������������У�����ţ�
��
�ټ�ͥ��ú�����˴ӡ�ú��������ú���ı仯
��ѧУ�������ȵ�λʹ�õĹ�¯Ҫ�÷���ķ�
��ȼ������п��Ե��ڿ�����С�Ľ���
�ܸ�ú¯����ʱ�����������ľ����ܵض���һ��
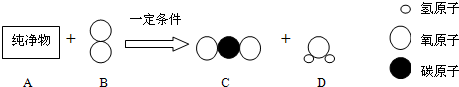
��4��ȼ��ȼ�ղ���֣���ɵĺ����
����ʹȼ��ȼ�ղ������������٣��˷���Դ�����һ������������CO�����ʣ���Ⱦ����
��
��5��ú��ʯ�͡���Ȼ�����ִ�����Ҫ��ȼ�ϣ�ͬʱҲ����Ҫ�Ļ���ԭ�ϣ�ʯ�����ƹ�������
����
�仯��ú���ۺ����ù�������
��ѧ
�仯��