



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
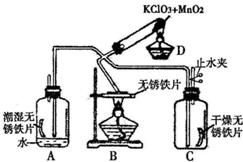 33��Ϊ����֤���������ڲ�ͬ�����µķ�Ӧ����������ͼ��ʾ����������̨����ȥ����ʵ�飬Dװ����O2����װ�ã�AΪ������ˮ��������O2��Ӧװ�ã�BΪ������Ƭ�ڼ�����������O2��Ӧװ�ã�Cװ������֤�ڸ���������O2������Ӧװ�ã�Cװ�����ȸ����������ش��������⣺
33��Ϊ����֤���������ڲ�ͬ�����µķ�Ӧ����������ͼ��ʾ����������̨����ȥ����ʵ�飬Dװ����O2����װ�ã�AΪ������ˮ��������O2��Ӧװ�ã�BΪ������Ƭ�ڼ�����������O2��Ӧװ�ã�Cװ������֤�ڸ���������O2������Ӧװ�ã�Cװ�����ȸ����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
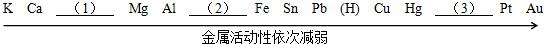
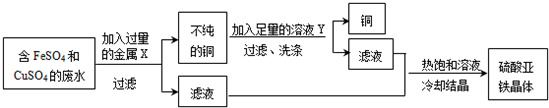
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

| ʵ������ | ʵ����� | ||||
| Aװ�� | Cװ�� | Eװ�� | �Ƿ��ж�����̼ | �Ƿ���һ����̼ | |
| ��ͬѧ | ʯ��ˮ ����� |
ʯ��ˮ û����� |
ʯ��ˮ û����� |
||
| ��ͬѧ | ʯ��ˮ ����� |
ʯ��ˮ ����� |
ʯ��ˮ ����� |
||
| ��ͬѧ | ʯ��ˮ û����� |
ʯ��ˮ û����� |
ʯ��ˮ ����� |
||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?���ţ�ij��ѧ��ȤС��ʹ����ͼ��ʾװ�ã���ij����ͭ�Ͻ�ijɷֽ��в�������ȡ����ϡ�������ձ��У��������м���14.0g�Ͻ���Ʒ��ʼ��ʱ������������ƽ�Ķ�����¼���±��У���ش��������⣺
��2013?���ţ�ij��ѧ��ȤС��ʹ����ͼ��ʾװ�ã���ij����ͭ�Ͻ�ijɷֽ��в�������ȡ����ϡ�������ձ��У��������м���14.0g�Ͻ���Ʒ��ʼ��ʱ������������ƽ�Ķ�����¼���±��У���ش��������⣺| ���ձ� | ���� ����� |
�������ۺ� 5���� |
�������ۺ� 12���� |
�������ۺ� 21���� |
�������ۺ� 40���� |
�������ۺ� 3Сʱ | |
| ������g�� | 28.5 | 169.7 | 183.6 | 183.5 | 183.4 | 183.3 | 183.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
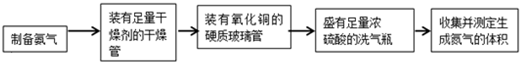
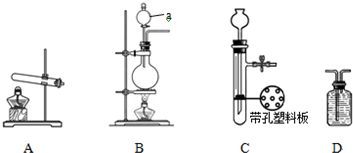
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� ��NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4 ��NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4 |
| ���� | �� B B |
Ũ��ˮ���������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com