
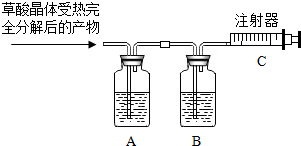
 ÓŠŠ©¾§Ģå°“±ČĄż“ųÓŠŅ»¶ØĮæµÄ½į¾§Ė®£¬“ųÓŠ½į¾§Ė®µÄ¾§ĢåŌŚŅ»¶ØĪĀ¶ČĻĀ»įĶŃČ„½į¾§Ė®£®²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©ŌŚ³£ĪĀĻĀŹĒŅ»ÖÖĪŽÉ«ĶøĆ÷¾§Ģ壮²ŻĖį¾§ĢåŹÜČČŹ±·Ö½āµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
ÓŠŠ©¾§Ģå°“±ČĄż“ųÓŠŅ»¶ØĮæµÄ½į¾§Ė®£¬“ųÓŠ½į¾§Ė®µÄ¾§ĢåŌŚŅ»¶ØĪĀ¶ČĻĀ»įĶŃČ„½į¾§Ė®£®²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©ŌŚ³£ĪĀĻĀŹĒŅ»ÖÖĪŽÉ«ĶøĆ÷¾§Ģ壮²ŻĖį¾§ĢåŹÜČČŹ±·Ö½āµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ·ÖĪö £Ø1£©øł¾Ż¼ÓČČŗóµÄ²śĪļĄ“·ÖĪö£¬AŹĒÓĆĄ“ĪüŹÕÉś³ÉµÄĖ®ÕōĘų”¢BŹĒĪüŹÕÉś³ÉµÄ¶žŃõ»ÆĢ¼£¬CŹĒŹÕ¼ÆÉś³ÉµÄŅ»Ńõ»ÆĢ¼£»
£Ø2£©øł¾ŻĢāŅā£¬AÖŠ²ŠĮōĘųĢåÖŠŗ¬ÓŠÉŁĮæµÄ¶žŃõ»ÆĢ¼£»
£Ø3£©øł¾Ż²Ī¼Ó·“Ó¦µÄ²ŻĖį¾§ĢåŅŌ¼°Éś³ÉĖ®µÄÖŹĮ棬ĄūÓĆ»Æѧ·½³ĢŹ½½ųŠŠ¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©ĪüŹÕÉś³ÉµÄĖ®ÕōĘųÓ¦øĆÓĆÅØĮņĖį£»ĪüŹÕÉś³ÉµÄ¶žŃõ»ÆĢ¼ĘųĢåÓĆÅØĒāŃõ»ÆÄĘČÜŅŗ£»×īŗóŹ£ÓąµÄŅ»Ńõ»ÆĢ¼ĘųĢå½ųČė×¢ÉäĘ÷£»¹ŹĢī£ŗÅØĮņĖį£»ĪüŹÕÉś³ÉµÄ¶žŃõ»ÆĢ¼ĘųĢ壻Ņ»Ńõ»ÆĢ¼£»
£Ø2£©ŅņŅŅŹĒøł¾ŻÉś³ÉµÄCO2Ą“Ēó½į¾§Ė®µÄÖŹĮ棬¶ųCO2Ņѱ»A×°ÖĆĪüŹÕĮĖŅ»²æ·Ö£¬ĖłŅŌŌö¼ÓµÄÖŹĮæbĘ«Š”£¬“Ó¶ųøł¾Ż»Æѧ·½³ĢŹ½Ėć³öµÄ½į¾§Ė®µÄÖŹĮæŅ²»įĘ«“ó£¬×īŗóĖć³ö½į¾§Ė®µÄÖŹĮæ·ÖŹżŅ²µ±Č»Ę«“óĮĖ£¬¹ŹĢī£ŗĘ«“ó£»
£Ø3£©½ā£ŗH2C2O4•xH2O$\frac{\underline{\;\;”÷\;\;}}{\;}$£Øx+1£©H2O+CO2”ü+CO”ü
90+18x 18£Øx+1£©
m a
Ōņ£ŗ$\frac{90+18x}{18£Øx+1£©}=\frac{m}{a}$
x=$\frac{m-5a}{a-m}$
Ōņ½į¾§Ė®µÄÖŹĮæ·ÖŹżĪŖ$\frac{18”Į\frac{m-5a}{a-m}}{90+18”Į\frac{m-5a}{a-m}}”Į100%$=$\frac{5a-m}{4m}”Į100%$
¹Ź“š°øĪŖ£ŗ$\frac{5a-m}{4m}”Į100%$
µćĘĄ ±¾ĢāŹōÓŚŠÅĻ¢ĢāµÄ漲飬½āĢāµÄ¹Ų¼üŹĒŅĄ¾ŻĢāÄæµÄŠÅĻ¢½įŗĻĻą¹ŲµÄÖŖŹ¶½ųŠŠ·ÖĪö£¬±¾ĢāŅŖĒóŹģĮ·ŌĖÓƶžŃõ»ÆĢ¼”¢Ņ»Ńõ»ÆĢ¼”¢Ė®µČµÄŠŌÖŹ²¢ÄÜøł¾Ż»Æѧ·½³ĢŹ½½ųŠŠ·ÖĪöŗĶ¼ĘĖć£®


 Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±łŃ©ČŚ»Æ | B£® | øɱłÉż»Ŗ | C£® | ĘūÓĶ»Ó·¢ | D£® | ľĢæČ¼ÉÕ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
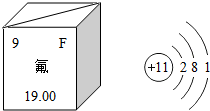 £Ø1£©·ś»ÆÄĘŹĒijŠ©ŃĄøąµÄĢķ¼Ó¼Į£¬ÄÜÓŠŠ§Ō¤·ĄČ£³Ż£®ČēĶ¼ŹĒ·śŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĻą¹ŲŠÅĻ¢¼°ÄĘŌ×Ó½į¹¹Ź¾ŅāĶ¼£¬Ōņ·śŌŖĖŲµÄŌ×ÓŗĖĶāµē×ÓŹżŹĒ9£¬ÄĘŌ×ÓŌŚ»Æѧ·“Ó¦ÖŠŅ׏§£ØĢī”°Ź§”±»ņ”°µĆ”±£©µē×Ó£®·ś»ÆÄĘæÉŅŌÓĆÄĘÓė·śĘų£ØF2£©·“Ó¦ÖĘµĆ£®·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ2Na+F2=2NaF£®
£Ø1£©·ś»ÆÄĘŹĒijŠ©ŃĄøąµÄĢķ¼Ó¼Į£¬ÄÜÓŠŠ§Ō¤·ĄČ£³Ż£®ČēĶ¼ŹĒ·śŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĻą¹ŲŠÅĻ¢¼°ÄĘŌ×Ó½į¹¹Ź¾ŅāĶ¼£¬Ōņ·śŌŖĖŲµÄŌ×ÓŗĖĶāµē×ÓŹżŹĒ9£¬ÄĘŌ×ÓŌŚ»Æѧ·“Ó¦ÖŠŅ׏§£ØĢī”°Ź§”±»ņ”°µĆ”±£©µē×Ó£®·ś»ÆÄĘæÉŅŌÓĆÄĘÓė·śĘų£ØF2£©·“Ó¦ÖĘµĆ£®·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ2Na+F2=2NaF£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ź³ÓĆ”°µŲ¹µÓĶ”±¶ŌČĖĢåÓŠŗ¦ | |
| B£® | Ćŗ”¢ŹÆÓĶ”¢ĢģČ»Ęų¶¼ŹōÓŚæÉŌŁÉśÄÜŌ“ | |
| C£® | ĪŖ±£ÕĻŹ³Ę·°²Č«£¬¶Å¾ųŹ¹ÓĆČĪŗĪŹ³Ę·Ģķ¼Ó¼Į | |
| D£® | ĪŖĢįøßÅ©×÷Īļ²śĮ棬Ӧ“óĮæŹ©ÓĆ»Æ·Ź |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹ūÖ | B£® | Å£ÄĢ | C£® | æóČŖĖ® | D£® | Ę”¾Ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Įņ | B£® | ľĢæ | C£® | ŗģĮ× | D£® | ĄÆÖņ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com