
��������װ��ͼ�ش����⡣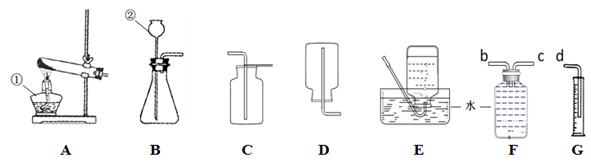
��д���������ƣ��� ��19�� ������20�� ��
��ʵ����������غͶ������̵Ļ������ȡ�����Ļ�ѧ����ʽΪ ��21�� ����ȡ���ռ�������װ������ǣ�����ĸ�� ��22�� ��������װ���ռ�����ʱ�ж������Ѿ������ķ����� ��23�� ��ͨ����ˮ���ⶨ��Ӧ�����������������װ�õ�����˳���ǣ�������O2 ��b��c��d���뽫Fװ���ڵ�һ�����ܽ������졢�������� ��24�� ��
��ֻ����Dװ���ռ�������Ӧ�þ��е������� ��25�� ��
����������ʵ���Ƶ�0.08g��������ֽ������ص����ʵ���Ϊ ��26�� mol�����ݻ�ѧ����ʽ��ʽ���㣩��
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(4.5��)ʵ���ҳ���������װ����������Ʊ�������ʵ�顣��ش�




A B C D E
��ָ��������������ƣ�a ��
����ʹ�ù���������Һ�Ͷ���������ȡ������Ӧѡ��ķ���װ���� ������ĸ������Ӧԭ���û�ѧ����ʽ��ʾΪ ��
�ǿ���ʹ��Cװ���ռ����������������������������� ���տ�ʼ�����ݲ��������ռ�����ԭ���� ��ʵ�����ʱ��Ӧ�ȴ�ˮ�����Ƴ������ܣ���Ϩ��ƾ��ƣ���ԭ���� ��������ѡ�� ������ĸ��װ�����ռ����������������������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(9��)��ͼ��ʵ�����г��õļ���װ�ã��ش��������⣺
A B C D E
��1��д���б�����������ƣ��� ���� ��
��2����������B��Cװ�õ���Ͽ�����ȡ��һ�������� ��
д����ȡ������Ļ�ѧ����ʽ ��
Ϊ��ֹ���ɵ�����ӳ���©���ݳ������ӵ�Һ��ֱ�� ��
��3�����ijͬѧҪ������غͶ���������ȡ�ϴ�����������ѡ������װ���� ��
��4�����������ȫ�ֽ�������ڷ�Ӧ��Ĺ�������ȡ���Ȼ��أ��ɾ�����������������
�ܽ� �� �� ����ɵõ��������Ȼ��ع��塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��9�֣� ������ͼ�ش����⣺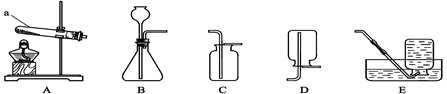
��l��ͼ��a���������� ��
��2���ù���������ȡ�����Ļ�ѧ����ʽΪ ���ռ��ϴ������������ѡ�õ�װ�� ������ĸ����ͬ����
��3���ô���ʯ��ϡ������ȡ������̼ʱ����ѡ�õķ���װ���� ����Ӧ�Ļ�ѧ����ʽΪ ��
��4����ͼ��ʾװ���ж�����;������˵����ȷ���� ��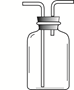
| A����ȥ�����е�ˮ��������ƿ��ʢ��Ũ���� |
| B�����������̼���塪��ƿ��ʢ������������Һ |
| C�����ſ������ռ��������������װ�õ�b��ͨ�� |
| D������ˮ���ռ���������ƿ����װ��ˮ�������b��ͨ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣�ij����С���ͬѧ������װ��̽��CO2��ʵ�����Ʒ���
��1����ͬѧ�ô���ʯ��ϡ���ᷴӦ��ȡCO2��Ӧ��ѡ�õķ���װ���� ���ռ�װ���� ������Ӧ�Ļ�ѧ����ʽΪ�� ��
��2�����ȹ���̼�����ƻ����̼����臨��ܲ���CO2���䷽��ʽ�ֱ��ǣ�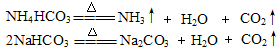
��ͬѧ���ü���̼�����Ƶķ�����ȡCO2��Ӧ��ѡ�õķ���װ���� ��
����ѡ��̼�������ȡCO2�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣���ͼΪʵ���ҳ��õ�ʵ��װ�ã��ش��й����⡣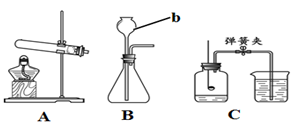
��1������b�������� ��
��2���ø������������ѡ��ķ���װ���� ����Ӧ�Ļ�ѧ����ʽΪ ���ռ����������� ����
��3��Cװ�����ڲⶨ�����������ĺ�������ֹˮ�к�۲쵽�������� ��ȷ��ʵ����ȷӦ��ȡ�Ĵ�ʩ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣����������ʵ��װ��ͼ�ش����⣺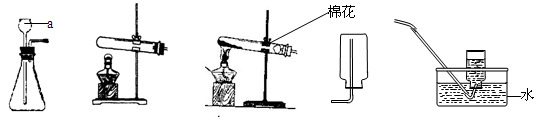
A B C D E
��1��д�����a���������ƣ�a ____________
��2���ø��������ȡ������Ӧѡ�õķ���װ�ú��ռ�װ����___ _____�����ţ�����������Ӧ�Ļ�ѧ����ʽΪ:__________ __ _________________��
��3��ʵ������ȡ������һ�㲽���У���װҩƷ �ڼ�������� �۹̶�װ�� �ܼ���
��Ϩ��ƾ��� �ްѵ��ܴ�ˮ�����ó� ���ռ����塣��ȷ��ʵ����������ǣ� ��
| A���٢ڢۢܢߢݢ� | B���ڢ٢ۢܢߢޢ� |
| C���٢ڢۢܢߢޢ� | D���ڢ٢ۢܢߢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(11��)��ͼ�г���ʵ������һЩʵ��װ�ã���ش��й����⣺ 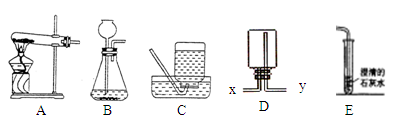
��1��װ��C�ɵ��ܡ�����ƿ�� �������������ƣ���ɡ�
��2��ʵ������H2O2��MnO2��ȡO2�Ļ�ѧ����ʽΪ ��ѡ�õķ���װ���� ������ţ���ͬ��������KClO3��MnO2��ȡO2����ѡ�õķ���װ���� ���ռ�װ�ÿ��� ��
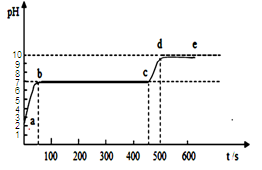
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ ��������̼����ͨ��E�У������������ǣ� ����ʯ��ʯ��ϡ������ȡ������̼ʱ��ѡ�õķ���װ���� ���������������ݳ���÷�Ӧ����Һ��pH��ͼ��a����ʾ���������Һ������̼������Һ����������ȷ�ⶨ��Һ��pH����ҺpH��ʱ��ı仯��ͼ������ʾ�������꣺pH�������꣺ʱ�䣩��
��д��bc�η�����Ӧ�Ļ�ѧ����ʽ�� �� cd ��������ԭ����: ��
��4��ʵ������ȡH2ʱ����ѡ��װ��D�ռ�H2����Ӧ�� �ڽ�������x��y����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���������ʵ������ȡ����ij���װ�ã��ش��й����⡣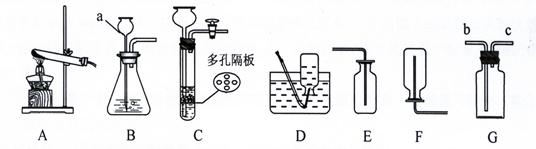
��1��д��ͼ�б�����ĸ���������ƣ�a ��
��2����A��E��Ͽ���ȡһ�����壬��Ӧ�Ļ�ѧ����ʽ
��
��3��ijͬѧ����Gװ�ô���Dװ�ã�����ˮ���ռ�һ�����������壬��ͬѧʹ��Gװ�õķ����� ��
��4��ʵ�������ÿ�״�����Һ�����������ȡ���壬�ɽ�Bװ�øĽ�ΪCװ�ã�����������ſ�״���壩�����ŵ��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com