
 4Al+3O2”ü£»£Ø4£©acd£®
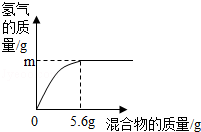
4Al+3O2”ü£»£Ø4£©acd£® 4Al+3O2”ü£»£Ø4£©Čō»ģŗĻĪļČ«ŹĒĢś£¬ŌņĢśµÄÖŹĮæĪŖ5.6 g£¬Ōņøł¾Ż»Æѧ·“Ó¦µÄ·½³ĢŹ½£ŗFe+2HCl=FeCl2+H2”üæɼĘĖć³ö“ĖŹ±²śÉśĒāĘųµÄÖŹĮæµČÓŚ0.2 g£¬Ķ¬ĄķæɼĘĖć³ö5.6 gAlÓėŃĪĖį·“Ó¦²śÉśĒāĘųµÄÖŹĮæ“óÓŚ0.2 g£¬5.6gŠæÓėŃĪĖį·“Ó¦²śÉśĒāĘųµÄÖŹĮæŠ”ÓŚ0.2 g£¬ĶŗĶŃĪĖį²»·“Ó¦£®a”¢Čō»ģŗĻĪļĪŖZn”¢Al£¬mæÉÄܵČÓŚ0.2g£¬¹ŹaÕżČ·£»b”¢Čō»ģŗĻĪļĪŖZn”¢Cu£¬ŌņmŅ»¶ØŠ”ÓŚ0.2g£¬¹Źb“ķĪó£»c”¢µ±»ģŗĻĪļĪŖFe”¢AlŹ±£¬ŅņĻąĶ¬ÖŹĮæµÄĀĮĻūŗÄĻ”ŃĪĖįµÄÖŹĮæ±ČĢśĻūŗÄŃĪĖįµÄÖŹĮæ“󣬶ų5.6 gĢśŹ±£¬ĻūŗÄŃĪĖįµÄÖŹĮæĪŖ7.3 g£¬ĖłŅŌ»ģŗĻĪļĪŖFe”¢AlŹ±£¬ĻūŗÄŃĪĖįµÄÖŹĮæ“óÓŚ7.3 g£¬øł¾ŻÖŹĮæ·ÖŹż¹«Ź½æÉÖŖĖłŠčŃĪĖįČÜÖŹÖŹĮæ·ÖŹżŅ»¶Ø“óÓŚ7.3%£¬¹ŹcÕżČ·£»d”¢Čō»ģŗĻĪļĪŖFe”¢Cu£¬mĪŖ0.1g£¬Éś³É0.1gĒāĘųŠčŅŖĢśµÄÖŹĮæŹĒ2.8g£¬Ōņ»ģŗĻĪļÖÖĢśµÄÖŹĮæ·ÖŹżĪŖ
4Al+3O2”ü£»£Ø4£©Čō»ģŗĻĪļČ«ŹĒĢś£¬ŌņĢśµÄÖŹĮæĪŖ5.6 g£¬Ōņøł¾Ż»Æѧ·“Ó¦µÄ·½³ĢŹ½£ŗFe+2HCl=FeCl2+H2”üæɼĘĖć³ö“ĖŹ±²śÉśĒāĘųµÄÖŹĮæµČÓŚ0.2 g£¬Ķ¬ĄķæɼĘĖć³ö5.6 gAlÓėŃĪĖį·“Ó¦²śÉśĒāĘųµÄÖŹĮæ“óÓŚ0.2 g£¬5.6gŠæÓėŃĪĖį·“Ó¦²śÉśĒāĘųµÄÖŹĮæŠ”ÓŚ0.2 g£¬ĶŗĶŃĪĖį²»·“Ó¦£®a”¢Čō»ģŗĻĪļĪŖZn”¢Al£¬mæÉÄܵČÓŚ0.2g£¬¹ŹaÕżČ·£»b”¢Čō»ģŗĻĪļĪŖZn”¢Cu£¬ŌņmŅ»¶ØŠ”ÓŚ0.2g£¬¹Źb“ķĪó£»c”¢µ±»ģŗĻĪļĪŖFe”¢AlŹ±£¬ŅņĻąĶ¬ÖŹĮæµÄĀĮĻūŗÄĻ”ŃĪĖįµÄÖŹĮæ±ČĢśĻūŗÄŃĪĖįµÄÖŹĮæ“󣬶ų5.6 gĢśŹ±£¬ĻūŗÄŃĪĖįµÄÖŹĮæĪŖ7.3 g£¬ĖłŅŌ»ģŗĻĪļĪŖFe”¢AlŹ±£¬ĻūŗÄŃĪĖįµÄÖŹĮæ“óÓŚ7.3 g£¬øł¾ŻÖŹĮæ·ÖŹż¹«Ź½æÉÖŖĖłŠčŃĪĖįČÜÖŹÖŹĮæ·ÖŹżŅ»¶Ø“óÓŚ7.3%£¬¹ŹcÕżČ·£»d”¢Čō»ģŗĻĪļĪŖFe”¢Cu£¬mĪŖ0.1g£¬Éś³É0.1gĒāĘųŠčŅŖĢśµÄÖŹĮæŹĒ2.8g£¬Ōņ»ģŗĻĪļÖÖĢśµÄÖŹĮæ·ÖŹżĪŖ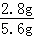 ”Į100%=50%£¬¹ŹdÕżČ·£»
”Į100%=50%£¬¹ŹdÕżČ·£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Zn | B£®Cu | C£®Mg | D£®Ag |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼×£¾ŅŅ£¾¶”£¾±ū | B£®±ū£¾¶”£¾ŅŅ£¾¼× | C£®ŅŅ£¾¶”£¾±ū£¾¼× | D£®±ū£¾ŅŅ£¾¶”£¾¼× |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®2.7g | B£®2.8g | C£®5.4g | D£®5.6g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®X£¾Y£¾Z | B£®Y£¾Z£¾X | C£®Y£¾X£¾Z | D£®X£¾Z£¾Y |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
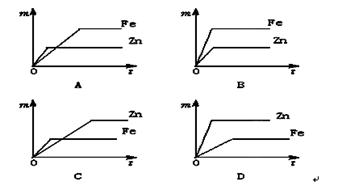
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
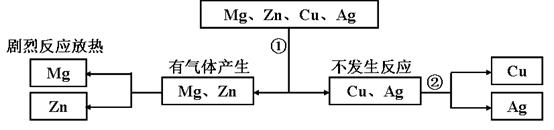
| A£®¢ŁĻ”ĮņĖį¢ŚĮņĖįĶ | B£®¢ŁĻ”ŃĪĖį¢ŚĮņĖįŠæ |
| C£®¢ŁĻ”ŃĪĖį¢ŚĮņĖįŃĒĢś | D£®¢ŁĻ”ĮņĖį¢ŚĀČ»ÆĆ¾ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com