
科目:初中化学 来源: 题型:
| 序号 | 加入稀盐酸质量(g) | 剩余固体质量(g) |
| 第1次 | 10 | 5.5 |
| 第2次 | 10 | m |
| 第3次 | 10 | 1.2 |
| 第4次 | 10 | 1.2 |
| ||
查看答案和解析>>
科目:初中化学 来源: 题型:
| 序号 | 第一次 | 第二次 | 第三次 | 第四次 |
| 加入稀盐酸质(克) | 10 | 10 | 10 | 10 |
| 剩余固体质量(克) | 5.5 | M | 1.2 | 1.2 |
查看答案和解析>>
科目:初中化学 来源: 题型:
| 序号 | 加入稀盐酸质量(g) | 剩余固体质量(g) |
| 第1次 | 10 | 5.5 |
| 第2次 | 10 | m |
| 第3次 | 10 | 1.2 |
| 第4次 | 10 | 1.2 |
| ||
查看答案和解析>>
科目:初中化学 来源: 题型:
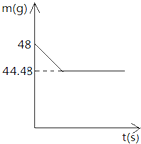 小强同学前往当地的石灰石矿区进行调查,他取回了若干块矿石样品,对样品中碳酸钙的质量分数进行检测,采用的办法如下:取用8g这种石灰石样品,把40g稀盐酸充分反应(已知石灰石样品中含的杂质不溶于水,不与盐酸反应),测得烧杯中的反应剩余物的质量(m)与反应时间(t)的关系如图所示.试计算:
小强同学前往当地的石灰石矿区进行调查,他取回了若干块矿石样品,对样品中碳酸钙的质量分数进行检测,采用的办法如下:取用8g这种石灰石样品,把40g稀盐酸充分反应(已知石灰石样品中含的杂质不溶于水,不与盐酸反应),测得烧杯中的反应剩余物的质量(m)与反应时间(t)的关系如图所示.试计算:查看答案和解析>>
科目:初中化学 来源:2007年北京市顺义区中考化学二模试卷(解析版) 题型:解答题
| 序号 | 第一次 | 第二次 | 第三次 | 第四次 |
| 加入稀盐酸质(克) | 10 | 10 | 10 | 10 |
| 剩余固体质量(克) | 5.5 | M | 1.2 | 1.2 |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com