
 ��ѧ��ʦָ��ij��ѧ��ȤС�飬�ⶨ�����ǵ���Ҫ�ɷ�̼��Ƶ��������������ǽ�1��2��������뵽l2g�������У�����������̼����������ͼ��ʾ�������輦�����е������ɷֲ������ᷢ����Ӧ��
��ѧ��ʦָ��ij��ѧ��ȤС�飬�ⶨ�����ǵ���Ҫ�ɷ�̼��Ƶ��������������ǽ�1��2��������뵽l2g�������У�����������̼����������ͼ��ʾ�������輦�����е������ɷֲ������ᷢ����Ӧ������ ��1������ͼ���ҳ����ɶ�����̼��������
��2�����ݻ�ѧ����ʽ�Ͷ�����̼�������������μӷ�Ӧ��̼��Ƶ�������Ȼ���������������
��� �⣺��1����ͼ�п��Կ�����12g�����������ᷴӦ�����ɶ�����̼��������4.4g��
��2���輦������̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
$\frac{100}{x}$=$\frac{44}{4.4g}$
x=10g
��������̼��Ƶ�����������$\frac{10g}{12g}$��100%=83.3%��
�˴�ΰ����1��4.4��
��2����Ʒ��̼��Ƶ�����������83.3%��
���� ���������������뻯ѧ����ʽ���ۺϼ��㣬����Ҫ��ȷд������ʽ���ٸ���������ϸ����������ϵ��������㣬������⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �㵽Һ�� | B�� |  ��ȡ��� | C�� |  ��ȼ�ƾ��� | D�� |  �μ�Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 |  |  |  |
| ����ƿ�й�$\frac{1}{4}$��ˮ | ����ƿ�й�$\frac{3}{4}$��ˮ | �������뵽����ƿ�����$\frac{1}{4}$�� | �������뵽����ƿ�����$\frac{3}{4}$�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
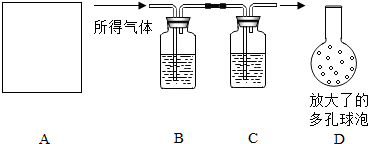 Ϊ�˲ⶨijʯ��ʯ�����Ĵ��ȣ������������ʲ����ᷴӦ����ijͬѧ���������̽���ʵ�飺�������ܽ���������������������NaOH��Һ�������������NaOH��Һ����������������Ĵ��ȣ�ʵ���������ȡ����������Ϊ10g��ʵ��װ����ͼ��ʾ��
Ϊ�˲ⶨijʯ��ʯ�����Ĵ��ȣ������������ʲ����ᷴӦ����ijͬѧ���������̽���ʵ�飺�������ܽ���������������������NaOH��Һ�������������NaOH��Һ����������������Ĵ��ȣ�ʵ���������ȡ����������Ϊ10g��ʵ��װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ⱦ���12��Ԫ����� | |
| B�� | ���Ⱦ�����Ԫ�ص���������Ϊ30% | |
| C�� | һ�����Ⱦ��������ɸ��Ⱦ���3��̼ԭ�ӡ�3����ԭ�ӡ�3����ԭ�Ӻ�3����ԭ�ӹ��� | |
| D�� | ���Ⱦ���C��O��N��Cl��Ԫ�������ȱ�Ϊ1��1��1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
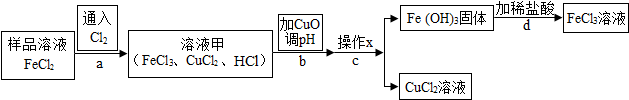
| Fe��OH��3 | Cu��OH��2 | |
| ��ʼ������pH | 1.9 | 4.7 |
| ������ȫ��pH | 3.2 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com