
��Ӣͬѧȡij��ʯ��ʯ��Ʒ12g���вⶨʵ�飬�ֽ�100gϡ�������μ���ʯ��ʯ��Ʒ�У����ʲ�����ˮҲ�����뷴Ӧ������ַ�Ӧ������������������������ʾ��
| ��1�� | ��2�� | ��3�� | ��4�� | ��5�� | |
| ����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
| ���������������/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
����
��1��m��ֵΪ�� ��g��
��2��12gʯ��ʯ��Ʒ��̼��Ƶ����������� ��g��
��3����Ӧ��ȫ��������Һ���Ȼ��Ƶ������������� ����
��д��������̣���������ȷ��0.1��
���㣺
���ݻ�ѧ��Ӧ����ʽ�ļ��㣻�й��������������ļ��㣮
ר�⣺
�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩��
������
��1�����ݼ�¼���ݿɷ��֣�20g��������ȫ��Ӧ���ɶ�����̼�����������1.1g����4��ʵ���м����������ɵĶ�����̼��4.4g��˵���˵�����ʵ����������ȫ��Ӧ�����Ƴ�m��ֵ��
��2�����ݶ�����̼�����������ʯ��ʯ��Ʒ��̼��Ƶ����������ɵ��Ȼ��Ƶ�������
��3���������������������ʵ��������������Ӧ��ȫ��������Һ���Ȼ��Ƶ�����������
���
�⣺��1�����ݼ�¼���ݿɷ��֣�20g��������ȫ��Ӧ���ɶ�����̼�����������1.1g����4��ʵ���м����������ɵĶ�����̼��4.4g��˵���˵�����ʵ����������ȫ��Ӧ�����Ƴ�m��ֵΪ3.3g��
��2����ʯ��ʯ��CaCO3������Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
x y 4.4g
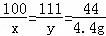
��ã�x=10g y=11.1g
��3����ȫ��Ӧ��������Һ���Ȼ��Ƶ����������ǣ� ��10.5%
��10.5%
�ʴ�Ϊ����1��3.3����2��10����3��10.5%��
������
������Ҫ������ѧ������ͼ�����ݣ����������������Լ��ݷ���ʽ�����������
��


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������ɡ���ȡ��Աȵķ����ó����н��ۣ����к������ǣ� ��
A.ͨ����������ϡ���ᷴӦ�ľ��ҳ̶����жϳ����Ļ�Ա���ǿ
B.ϡ�������������Ʒ�Ӧ���ȣ����кͷ�Ӧһ������
C.Na+��Mg2+��Cl-��������������Ϊ8���ɴ˵ó����ӵ�������������Ϊ8
D. ��ij��Һ�м�������ϡ���ᣬ�����������ټ��Ȼ�����Һ���а�ɫ�������֣���ԭ��Һ��һ������SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʵķ�������ȷ����( )
A����ˮ����̼��Ʒ�ĩ��̼���Ʒ�ĩ
B����ˮ��������粒�����������ƹ���
C�������ᱵ��Һ����ϡ�����ϡ����
D���÷�̪��Һ����ϡ�����ʳ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʢ��5g 16%��NaOH��Һ�Ķ��Թܷ���ʢ��10g 16%��CuSO4��Һ����ƿ���ͼ��ʾ����б��ƿֱ��ʹ����Һ��ֻ�ϣ���Ϻ��ܵ��ǣ�������

| �� | A�� | ������ɫ����0.98g |
| �� | B�� | ��ƿ���������������� |
| �� | C�� | ������Һ��Na2SO4������������9.5% |
| �� | D�� | ���õ�Na2SO4��Һ������ԭCuSO4��Һ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ļ��Ų����ߺ����������ƽ𣩺��н����������ɷֺ��Բ��ƣ���ijͬѧȡһ�������������߽��ϣ���������ϡ���ᣬ��ַ�Ӧ�ų�0.3gH2��ʣ���������Ϊ0.1g��
��1������Ʒ������������
��2������Ʒ�н������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ⶨij����������淋Ĵ��ȣ��������������Ƿ�����ͼ��ǩ�����С��ȡ15g�õ�����Ʒ�����Һ�����������Ȼ�����Һ��ַ�Ӧ���ˡ�ϴ�ӡ���ɣ���ó�������Ϊ23.3g����ͨ������ȷ���õ���������淋Ĵ����Ƿ����ǩ�����
����Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+BaCl2=BaSO4��+2NH4Cl�������ɷ�������ˮ�����μӷ�Ӧ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ⶨijCu﹣Zn�Ͻ���ͭ������������ijͬѧ��10g�úϽ���뵽ʢ��40g����ϡ������ձ��У���ַ�Ӧ����ձ���ʣ���������Ϊ49.9g��
��1������������������������������
��2������ԭ�Ͻ���ͭ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ���϶���ʳ����������Ҫ���ã����������ʵ��ǣ�������
| �� | A�� | NH4HCO3 | B�� | CO��NH2��2 | C�� | KNO3 | D�� | Ca��H2PO4��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڴ�����������ȷ����(����)
A���κλ�ѧ��Ӧ����Ҫ����
B�������ڷ�Ӧǰ���������������˱仯
C��ֻ�зֽⷴӦ����Ҫ����
D��ijЩ��ѧ��Ӧ�����ж��ִ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com