
| 实验次数 项目 | 第一次 | 第二次 | 第三次 | 第四次 |
| 所取石灰石样品的质量/g | 12 | 12 | 12 | 12 |
| 所加稀盐酸的质量/g | 25 | 35 | 50 | 70 |
| 生成二氧化碳气体的质量/g | 2.2 | 3.1 | 4.4 | 4.4 |
分析 (1)第1、2次比较可以发现,当稀盐酸的质量增加时,气体的质量也在增加,说明第1份中碳酸钙没有完全反应,稀盐酸完全反应.
(2)根据二氧化碳的质量和方程式计算碳酸钙的质量,进一步计算样品中碳酸钙的质量分数.
(3)根据二氧化碳的质量和方程式计算氯化钙的质量,进一步计算样品中氯化钙的质量分数
解答 解:(1)第1、2次比较可以发现,当稀盐酸的质量增加时,气体的质量也在增加,说明第1份中碳酸钙没有完全反应,稀盐酸完全反应,并且每消耗25g盐酸生成2.2g二氧化碳,第三次加入50g盐酸生成4.4g二氧化碳后,二氧化碳的质量不再增加,说明第三、四次实验样品里的碳酸钙就已反应完.
(2)设样品中碳酸钙的质量为x,生成氯化钙的质量为y.
CaCO3+2HCl=CaCl2+H20+CO2↑
100 111 44
x y 4.4g
$\frac{100}{x}$=$\frac{111}{y}$=$\frac{44}{4.4g}$
x=10g,y=11.1g
品中碳酸钙的质量分数是$\frac{10g}{12g}$×100%=83.3%
(3)第三次实验所得溶液溶质质量分数为$\frac{11.1g}{50g+10g-4.4g}$×100%=20.0%
故答案为:(1)三、四(2)83.3%
(3)20.0%
点评 本题主要考查学生运用所学化学知识分析和解决实际问题的能力,增加了学生分析问题的思维跨度,强调了学生整合知识的能力.


 阅读快车系列答案
阅读快车系列答案科目:初中化学 来源: 题型:填空题
 ”表示氢原子,“
”表示氢原子,“ ”表示氧原子.
”表示氧原子.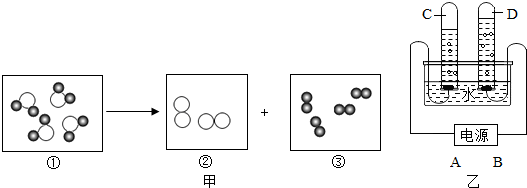
查看答案和解析>>
科目:初中化学 来源: 题型:选择题
| A. | 缺乏氧气 | |
| B. | 火柴棍的着火点升高了 | |
| C. | 缺乏可燃物 | |
| D. | 燃烧端下方的火柴棍温度不易达到着火点 |
查看答案和解析>>
科目:初中化学 来源: 题型:选择题
| A. | 13g | B. | 12.6g | C. | 10g | D. | 11.6g |
查看答案和解析>>
科目:初中化学 来源: 题型:填空题
查看答案和解析>>
科目:初中化学 来源: 题型:选择题
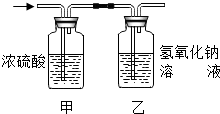 某有机物3.2g在氧气中充分燃烧,将生成的水蒸气、二氧化碳气体依次通过装置甲和乙 (假设每步吸收完全),称量通入气体前后装置的质量,数据如下:分析上表的数据,该有机物的化学式是( )
某有机物3.2g在氧气中充分燃烧,将生成的水蒸气、二氧化碳气体依次通过装置甲和乙 (假设每步吸收完全),称量通入气体前后装置的质量,数据如下:分析上表的数据,该有机物的化学式是( )| 装置甲 | 装置乙 | |
| 通气前质量 | 200g | 180g |
| 通气后质量 | 203.6g | 184.4g |
| A. | CH4 | B. | C2H4 | C. | C2H5OH | D. | CH3OH |
查看答案和解析>>
科目:初中化学 来源: 题型:选择题
| A. | ①②④⑤ | B. | ①②③④ | C. | ④⑤ | D. | ①②③ |
查看答案和解析>>
科目:初中化学 来源: 题型:解答题
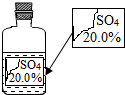 一次趣味化学活动中,王老师向同学们展示了一瓶标签受损的无色溶液,如图所示.要求同学们进行探究:确认这瓶溶液是什么溶液?
一次趣味化学活动中,王老师向同学们展示了一瓶标签受损的无色溶液,如图所示.要求同学们进行探究:确认这瓶溶液是什么溶液?| 物质 | MgSO4 | Na2SO4 | (NH4)2SO4 | H2SO4 |
| 溶解度 | 35.1g | 19.5g | 75.4g | 与水以任意比互溶 |
| 实验操作 | 实验现象 | 实验结论 |
| ①取该溶液少许于试管中,向其中滴加几滴NaOH(合理答案均可)溶液 | 溶液中有白色沉淀生成 | 猜想①成立 |
| ②用玻璃棒蘸取少许原溶液滴在pH试纸上,并跟标准比色卡对照 | 溶液pH小于7 | 猜想③成立 |
| 实验操作 | 实验现象 | 实验结论 |
| 取该溶液少许于试管中,向试管中加入少量的NaOH溶液并加热,将湿润的红色石蕊试纸放在试管口有刺激性气味的气体产生 | 有刺激性气味的气体产生,湿润的红色石蕊试纸变蓝 | 猜想④成立,该反应的化学方程式为(NH4)2SO4+2NaOH$\frac{\underline{\;\;△\;\;}}{\;}$Na2SO4+2NH3↑+2H2O |
查看答案和解析>>
科目:初中化学 来源: 题型:解答题
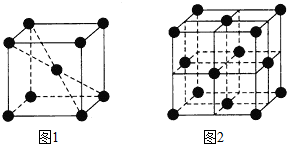 910℃以下纯铁晶体的基本结构单元如图1所示,910℃以上转变为图2所示的结构单元,在两种晶体中最邻近的铁原子间距离相同.
910℃以下纯铁晶体的基本结构单元如图1所示,910℃以上转变为图2所示的结构单元,在两种晶体中最邻近的铁原子间距离相同.查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com