

 ×100%¼“æÉĒóµĆѳʷ֊ĮņĖįÄʵÄÖŹĮæ·ÖŹż£»
×100%¼“æÉĒóµĆѳʷ֊ĮņĖįÄʵÄÖŹĮæ·ÖŹż£»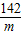 =
=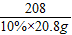
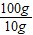 =14.2 g£»
=14.2 g£»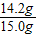 ×100%”Ö94.7%
×100%”Ö94.7%


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ½ĖÕŗéŌóĻŲĖ³ŗÓÕņÄŚĆ¢ĻõæóŹĒ»Ŗ¶«ĪØŅ»ĢŲ“óĆ¢Ļõæó£¬ŅŃĢ½Ć÷“¢Įæ×īøߣ¬ĒŅŌŖĆ÷·ŪĘ·Ī»øß”¢ÖŹĮæŗĆ£¬ÉīŹÜ¹śÄŚĶāæĶÉĢĒąķł£®ŌŖĆ÷·ŪŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬ČēĶ¼ŹĒÄ³Ę·ÅĘŌŖĆ÷·Ū°ü×°“üÉĻµÄ²æ·Ö±źĒ©£®ĪŖ²ā¶ØøĆŌŖĆ÷·ŪÖŠNa2SO4ŗ¬ĮæŹĒ·ń·ūŗĻ±źĒ©ŅŖĒ󣬰Ń15.0gѳʷ£Ø¼ŁÉčÖ»ŗ¬²»ČÜŠŌŌÓÖŹ£©¼ÓČėŅ»¶ØĮæµÄĖ®Čܽā£¬¹żĀĖµĆ100.0gĀĖŅŗ£®Č”10.0gĀĖŅŗ£¬¼ÓČė10%µÄĀČ»Æ±µČÜŅŗ20.8g£¬Ē”ŗĆĶźČ«·“Ó¦£®·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗNa2SO4+BaCl2=2NaCl+BaSO4”ż£®
½ĖÕŗéŌóĻŲĖ³ŗÓÕņÄŚĆ¢ĻõæóŹĒ»Ŗ¶«ĪØŅ»ĢŲ“óĆ¢Ļõæó£¬ŅŃĢ½Ć÷“¢Įæ×īøߣ¬ĒŅŌŖĆ÷·ŪĘ·Ī»øß”¢ÖŹĮæŗĆ£¬ÉīŹÜ¹śÄŚĶāæĶÉĢĒąķł£®ŌŖĆ÷·ŪŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬ČēĶ¼ŹĒÄ³Ę·ÅĘŌŖĆ÷·Ū°ü×°“üÉĻµÄ²æ·Ö±źĒ©£®ĪŖ²ā¶ØøĆŌŖĆ÷·ŪÖŠNa2SO4ŗ¬ĮæŹĒ·ń·ūŗĻ±źĒ©ŅŖĒ󣬰Ń15.0gѳʷ£Ø¼ŁÉčÖ»ŗ¬²»ČÜŠŌŌÓÖŹ£©¼ÓČėŅ»¶ØĮæµÄĖ®Čܽā£¬¹żĀĖµĆ100.0gĀĖŅŗ£®Č”10.0gĀĖŅŗ£¬¼ÓČė10%µÄĀČ»Æ±µČÜŅŗ20.8g£¬Ē”ŗĆĶźČ«·“Ó¦£®·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗNa2SO4+BaCl2=2NaCl+BaSO4”ż£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ½ĖÕŗéŌóĻŲĖ³ŗÓÕņÄŚĆ¢ĻõæóŹĒ»Ŗ¶«ĪØŅ»ĢŲ“óĆ¢Ļõæó£¬ŅŃĢ½Ć÷“¢Įæ×īøߣ¬ĒŅŌŖĆ÷·ŪĘ·Ī»øß”¢ÖŹĮæŗĆ£¬ÉīŹÜ¹śÄŚĶāæĶÉĢĒąķł£®ŌŖĆ÷·ŪŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬ČēĶ¼ŹĒÄ³Ę·ÅĘŌŖĆ÷·Ū°ü×°“üÉĻµÄ²æ·Ö±źĒ©£®ĪŖ²ā¶ØøĆŌŖĆ÷·ŪÖŠNa2SO4ŗ¬ĮæŹĒ·ń·ūŗĻ±źĒ©ŅŖĒ󣬰Ń15.0gѳʷ£Ø¼ŁÉčÖ»ŗ¬²»ČÜŠŌŌÓÖŹ£©¼ÓČėŅ»¶ØĮæµÄĖ®Čܽā£¬¹żĀĖµĆ100.0gĀĖŅŗ£®Č”10.0gĀĖŅŗ£¬¼ÓČė10%µÄĀČ»Æ±µČÜŅŗ20.8g£¬Ē”ŗĆĶźČ«·“Ó¦£®·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗNa2SO4+BaCl2=2NaCl+BaSO4”ż£®
½ĖÕŗéŌóĻŲĖ³ŗÓÕņÄŚĆ¢ĻõæóŹĒ»Ŗ¶«ĪØŅ»ĢŲ“óĆ¢Ļõæó£¬ŅŃĢ½Ć÷“¢Įæ×īøߣ¬ĒŅŌŖĆ÷·ŪĘ·Ī»øß”¢ÖŹĮæŗĆ£¬ÉīŹÜ¹śÄŚĶāæĶÉĢĒąķł£®ŌŖĆ÷·ŪŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬ČēĶ¼ŹĒÄ³Ę·ÅĘŌŖĆ÷·Ū°ü×°“üÉĻµÄ²æ·Ö±źĒ©£®ĪŖ²ā¶ØøĆŌŖĆ÷·ŪÖŠNa2SO4ŗ¬ĮæŹĒ·ń·ūŗĻ±źĒ©ŅŖĒ󣬰Ń15.0gѳʷ£Ø¼ŁÉčÖ»ŗ¬²»ČÜŠŌŌÓÖŹ£©¼ÓČėŅ»¶ØĮæµÄĖ®Čܽā£¬¹żĀĖµĆ100.0gĀĖŅŗ£®Č”10.0gĀĖŅŗ£¬¼ÓČė10%µÄĀČ»Æ±µČÜŅŗ20.8g£¬Ē”ŗĆĶźČ«·“Ó¦£®·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗNa2SO4+BaCl2=2NaCl+BaSO4”ż£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com