
���±�����ʵ�飬�й�˵��������������ǣ�������
������NaOH��Һ�еμ�CuSO4��Һ ������BaCl2��Һ�еμ�CuSO4��Һ
���� �� ��ɫ����
��ѧ����ʽ CuSO4+2NaOH=Cu��OH��2��+Na2SO4 ��
A��������ɫ����
B���ڷ�Ӧ�Ļ�ѧ����ʽΪ��CuSO4+BaCl2=BaSO4��+CuCl2
C��������Ӧ�����ڸ��ֽⷴӦ
D��ֻҪ�г������ɵķ�Ӧ�����ڸ��ֽⷴӦ
�����㡿�εĻ�ѧ���ʣ����ֽⷴӦ���䷢����������
��ר�⡿�������� ��ѧ���ϣ�
�������������������ƺ�����ͭ��Ӧ��������ɫ��������ͭ�����������ƣ��Ȼ���������ͭ��Ӧ�����ɰ�ɫ�����ᱵ������֪ʶ���з�����
����𡿽⣺A���������ƺ�����ͭ��Ӧ��������ɫ��������ͭ��������A��ȷ��
B���Ȼ���������ͭ��Ӧ�����ɰ�ɫ�����ᱵ��������ѧ����ʽΪ��CuSO4+BaCl2=BaSO4��+CuCl2����B��ȷ��
C��ͨ��������������Ӧ�����ϸ��ֽⷴӦ����������C��ȷ��
D��������̼���������ƻ�����̼��Ƴ�����ˮ�������ڸ��ֽⷴӦ����D����
��ѡ��D��
��������������Ҫ�������йظ��ֽⷴӦ��Ӧ���о����ѶȲ���ע�����ո��ֽⷴӦ����д�������ɽ��
��


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ش����л�ѧ���������³��������ʣ�A������ B����Ȼ�� C��ʯī D��ϡ�����壮�밴Ҫ����գ�����ĸ��ţ�
��1�����������䡱���ƹ�ʹ�õ����ȼ����������
��2�������ƹ�������������������ھ�ˮ�������������������䡱���������Ȼ����ȼ�յIJ�����ˮ�Ͷ�����̼���������ȼ�ϣ��ʴ�Ϊ����
��2��ϡ�������ֳƶ������壬��ѧ�����ȶ������������������ʷ�����ѧ��Ӧ��������;�������ֵ��Դ�����������ʴ�Ϊ����
��3��ʯī���е����ԣ��������ɵ�صĵ缫���ʴ�Ϊ����
��4��������ʹС�Ŀ����ۼ��ɴ�Ŀ�����Ȼ�����������������ˮ���ã��ʴ�Ϊ����
��5��ʯī���л���У���ֽ�ϻ��������ºۼ���������Ǧ��о���йʴ�Ϊ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������۵���ϵ�ǻ�ѧ���ص�˼ά��ʽ�����ж��ں��������۽����д�����ǣ�������
A������ƹ���������ˮ���ܹ�����������Ϊ�����������ͱ��
B��������ѹ����Һ��������Ϊ�����ʵļ����С
C����ͬ�Ļ����в�ͬ����ζ������Ϊ��ͬ�ķ������ʲ�ͬ
D�����������ܵ��������̵�Σ��������Ϊ�����ڲ��ϵ��˶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
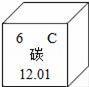
��1����ͼΪԪ�����ڱ��е�һ������˵������ȷ����������
A��̼Ԫ�����ڷǽ���Ԫ�� B��̼ԭ�Ӻ���������Ϊ6
C��̼Ԫ�ص�ԭ�ӽṹʾ��ͼΪ D��̼�����ԭ������Ϊ12.01
D��̼�����ԭ������Ϊ12.01
��2��ú����������Ȼ������Ϊ��ʯȼ�ϣ�����ʹ����Ȼ����ȼ�ϣ��ŷ���Ⱦ����٣�д����Ȼ��ȼ�յĻ�ѧ����ʽ����
��3������̼����������ܺġ�����Ⱦ�����ŷţ���Ҫ��Ϊ�˼����������ѧʽ�����ŷ����������������ϡ���̼���á�����������
���ᳫ���͵��Ӻؿ� �ڴ�����չ�������� �����ƺͿ�������Դ
���ᳫʹ��һ����ľ�� ����ǿ������Ȼ�ɹ�
��4��������̼�Ͱ�����NH3���ڸ��¡���ѹ�·�Ӧ����������[CO��NH2��2]��ˮ����Ӧ�Ļ�ѧ����ʽ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧϰ��ѧ���õ�˼ά������������Һ���о�һ�Ե��ص���������������ҺӦ���ǣ�������
A����ɫ��
B���ϲ���Һ���²���Һһ����
C�������
D��ˮ�֡��¶Ȳ���ʱ�����Ǻ�ˮ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼʾ��Ϣ���û�ѧ������գ�
��1��д�������н���Ԫ�ص�Ԫ�ط�������
��2��д����Һ�д������ڵ������ӵķ�������
��3������ܼ�����Ԫ�صĻ��ϼ��� ����
����
��4��д������Һ�����ʵĻ�ѧʽ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������ijNaCl��Һ�м���������AgNO3��Һ������AgCl������������ԭNaCl��Һ����������ԭNaCl��Һ�����ʵ�����������Ϊ������������������0.1%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ����Ӧ�Ľ��Ͳ�һ�µ���( )
| ѡ�� | ��ʵ | ���� |
| A | 50mLˮ��50mL�ƾ���Ϻ�����С��100mL | ���Ӽ��м�� |
| B | �����ͳ�����O3�����ʲ���ȫ��ͬ | �������ʵķ��Ӳ�ͬ |
| C | ϡ������Ļ�ѧ�����ȶ� | ԭ�ӵ��������Ӵﵽ�ȶ��ṹ |
| D | ͭ���ڿ����в�ȼ�գ�����ͭ�ڿ����п���ȼ�� | ���ʵ����������Ӧ�ܷ���� |
A��A B��B C��C D��D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڻ�ѧ��ӦA+B=C+D������˵���У���ȷ���ǣ�������
A����A��D�����������B��C���������������
B����������C��D�ֱ����κ�ˮ����÷�Ӧһ�����кͷ�Ӧ
C����A�ǿ����ԼB�ǿ������Σ���C��D�����ܶ��dz���
D����A��C���ǵ��ʣ�B��D���ǻ������÷�Ӧһ�����û���Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com