��2010?���գ���ʽ̼��ͭ[Cu
2��OH��
2CO
3]���׳ƿ�ȸʯ��ͭ�̣���ͭ������е�������������̼��ˮ��Ӧ���������ʣ���ʽ̼��ͭ���ȷ����ֽ⣬�����������������ʦΪ����ͬѧ��̽����ʽ̼��ͭ������ȫ�ֽ��IJ����ͬѧ����������������ҩƷ����Ƥ�����ɣ����ԣ�������֪��ʯ�ҵ���Ҫ�ɷ��������ƺ��������ƣ����������ʵ�飺
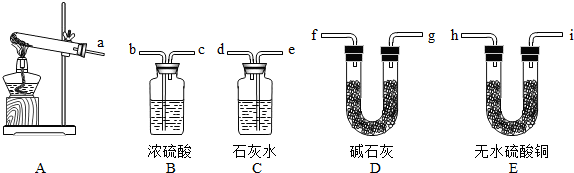
��1��ʵ��ǰ������A�з�Ӧ���������ԵIJ�����
������û��ˮ�У����ֽ����Թ���ڣ��۲쵼�ܿڲ������ݣ���ȴ����������һ��ˮ����˵�����������ã�
������û��ˮ�У����ֽ����Թ���ڣ��۲쵼�ܿڲ������ݣ���ȴ����������һ��ˮ����˵�����������ã�
��
��2��Ϊ����֤�����е����壬�罫����������ͨ��װ��
C
C
����װ����ţ���ͬ����������
ʯ��ˮ�����
ʯ��ˮ�����
����֤���������ж�����̼��������е�����ʹ
E
E
װ���е����ʱ���ɫ����֤����������
ˮ
ˮ
��
��3����һ���������������֤������ʹʵ�����ѧ������Ӧ�ڣ�2������ѡ������װ���м�����
B
B
װ�ã�Ŀ����
���������е�ˮ
���������е�ˮ
��
��4����֤�����еĺ�ɫ���壬Ӧѡ��
A
A
װ�ý���ʵ�飬����Ҫһ�ֹ��巴Ӧ����
ľ̿��
ľ̿��
��д���ƣ���
��5����ʽ̼��ͭ���ȷֽ�Ļ�ѧ����ʽ
Cu
2��OH��
2CO
32CuO+CO
2��+H
2O
Cu
2��OH��
2CO
32CuO+CO
2��+H
2O
-���÷�Ӧ��������
�ֽⷴӦ
�ֽⷴӦ
��




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�