
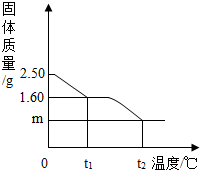
 С��ͬѧΪ�о�����������Ⱥ��������ʵ���ɣ���������ʵ�飺ȡ2.50g��������
С��ͬѧΪ�о�����������Ⱥ��������ʵ���ɣ���������ʵ�飺ȡ2.50g�������� CuSO4+XH2O����
CuSO4+XH2O���� CuSO4+xH2O����
CuSO4+xH2O����
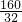 =5��
=5�� =0.64g��
=0.64g��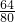 =0.80g��
=0.80g��

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
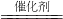 ______+6H2O��
______+6H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
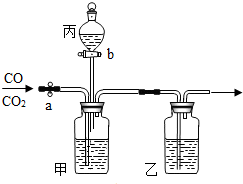 ��ʵ��������ͼ��ʾװ�ý���CO��CO2�ķ�������ֻ��ϡ���ᡢŨ���ᡢʯ��ˮ����Ҫ����д���пհ�
��ʵ��������ͼ��ʾװ�ý���CO��CO2�ķ�������ֻ��ϡ���ᡢŨ���ᡢʯ��ˮ����Ҫ����д���пհ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1������5�£����и�ѧУ������ʵ��������飮С��ͬѧ���е�ʵ�鿼���ǡ���ϡ��������������ƣ�NaOH����Һ��̼���ƣ�Na2CO3����Һ�����ⶨ̼������Һ��pH����
��С��ͬѧ��ʵ���¼���±����벹��������
| �������� | �����¼ | ��Ӧ�Ļ�ѧ����ʽ |
| ȡ��֧�Թܣ��ֱ����Թ��м���2mL��Ʒ1��2���������е������� | ��Ʒ1������������ | ______ |
| ��Ʒ2����Һ�г������� | ______ |
����pH��ֽ�ⶨ̼������Һ�����ȣ�������������Ҫ���裺______��
��2����ʵ��̨������ƿδ����ǩ����Һ����֪�ֱ���̼������Һ������������Һ��ϡ���ᣮΪ������������Һ������ʦָ���£���ȤС���ͬѧ����������Һ��A��B��C���б�ţ�Ȼ��ֱ��ȡ������Ϊ��Ʒ���뵽��֧�Թ��У���������ͼ��ʾ��̽�����
����һ�У�C����������A��B��Һ����ɫ��ɺ�ɫ��
������У�A��B��Һ����ɫ�ɺ�ɫ�����ɫ����B��Һ��������ð����
�ٸ�������ʵ�������֪��B��C��Һ�ֱ���______��______��
��ijС��ͬѧ�ڽ��в���һʵ��ʱ�����쳣������A��Һ�м����̪��Һʱ����Һ��ɫ�ȱ�ɺ�ɫ����Ѹ�ٱ����ɫ����ʦָ��������Ϊ��ҺŨ�ȹ�����ɵģ����ţ���ʦ�����Թܵ���Һ�м����������ᣬ�۲쵽A��Һ����ɫ�ֱ�ɺ�ɫ����ʱ��Һ�к��е�������Ҫ��______����̪���⣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | ����� | |
| ��ͬѧ | ȡpH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ����������Һմ��pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ� | pH��7 |
| ��ͬѧ | ��pH��ֱֽ�ӽ�������������Һ�У�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ� | pH��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
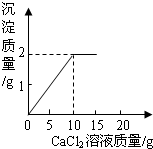 ��֪CaCl2+Na2CO3=CaCO3��+2NaCl����15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ��������ϵ��ͼ��ʾ��������Ӧ������ʹ��ˣ�������Һ��õ�����������Ƕ��٣�
��֪CaCl2+Na2CO3=CaCO3��+2NaCl����15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ��������ϵ��ͼ��ʾ��������Ӧ������ʹ��ˣ�������Һ��õ�����������Ƕ��٣��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com