
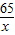 =
=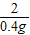
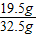 ×100%=60%
×100%=60% 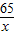 =
=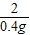
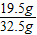 ×100%=60%
×100%=60% 

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����ѧ������ ���ͣ�022
(2004
����ɽ)С����ⶨCu��Zn�Ͻ�Cu��Ag�Ͻ���ͭ������������ʵ����ֻ�ṩһƿδ��������������ϡ����ͱ�Ҫ��������(1)
����Ϊ�ܲ����ͭ��������������________�Ͻ�(2)
С��ȡ�úϽ�ķ�ĩ32.5g���������ĸ������ַ�Ӧ�����ⶨ��������0.4g���壬������úϽ���ͭ������������(3)
���������������������������Ϊʵ��ʱ�����ṩ�Ͳ����������________(ѡ�����)��[
����]A
���μӷ�Ӧ�ĺϽ�����B
���μӷ�Ӧ��ϡ���������C
���μӷ�Ӧ��ϡ�����������ܶ�D
������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
(2004
����ɽ)С����ⶨCu��Zn�Ͻ�Cu��Ag�Ͻ���ͭ������������ʵ����ֻ�ṩһƿδ��������������ϡ����ͱ�Ҫ��������(1)
����Ϊ�ܲ����ͭ��������������________�Ͻ�(2)
С��ȡ�úϽ�ķ�ĩ32.5g���������ĸ������ַ�Ӧ�����ⶨ��������0.4g���壬������úϽ���ͭ������������(3)
���������������������������Ϊʵ��ʱ�����ṩ�Ͳ����������________(ѡ�����)��[
����]A
���μӷ�Ӧ�ĺϽ�����B
���μӷ�Ӧ��ϡ���������C
���μӷ�Ӧ��ϡ�����������ܶ�D
������������������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com