
| Ԫ������ | Ԫ�ط��� | ԭ�ӽṹʾ��ͼ | ���� ��ѧʽ |
���³�ѹ�µ��ʵ�״̬ | ���������� ��Ӧ��״�� |
| �� | F |  |
F2 | ��̬ ��̬ |
�ڰ�����ը |
| �� | Cl | 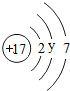 y= 8 8 |
Cl2 | ��̬ | ���ձ�ը |
| �� | Br | 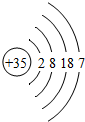 |
Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |  x= 53 53 |
I2 I2 |
��̬ | �������Ȼ�����Ӧ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | ԭ�ӽṹʾ��ͼ | ���ʻ�ѧʽ | ���³�ѹ��״̬ | ������������Ӧ |
| �� | F |  | F2 | ��̬ ��̬ | �ڰ�����ը |
| �� | Cl |   | Cl2 | ��̬ | ���ձ�ը |
| �� | Br |  | Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |  | I2 I2 | ��̬ | �������Ȼ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | ԭ�ӽṹʾ��ͼ | ���ʵ� ��ѧʽ |
���³�ѹ�� ״̬ |
������������Ӧ |
| �� | F |  |
F2 | ��̬ | �ڰ�����ը |
| �� | Cl |  |
Cl2 | ��̬ | ���ձ�ը |
| �� | Br |  |
Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |  |
I2 | ��̬ | �������Ȼ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ������ | Ԫ�ط��� | ԭ�ӽṹʾ��ͼ | ���� ��ѧʽ | ���³�ѹ�µ��ʵ�״̬ | ���������� ��Ӧ��״�� |
| �� | F |  | F2 | ______ | �ڰ�����ը |
| �� | Cl |  y=______ | Cl2 | ��̬ | ���ձ�ը |
| �� | Br |  | Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |  x=______ | ______ | ��̬ | �������Ȼ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ƚ���������ɻ�ʹ���ͷ�Ա�ø�������������±�����Ϣ���ش��й����⣺
| ���� | ���� | ԭ�ӽṹʾ��ͼ | ���ʻ�ѧʽ | ���³�ѹ��״̬ | ������������Ӧ |
| �� | F |
| F2 |
| �ڰ�����ը |
| �� | Cl |
| Cl2 | ��̬ | ���ձ�ը |
| �� | Br |
| Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |
|
| ��̬ | �������Ȼ�����Ӧ |
��1������������еĿո�������
��2�����Թ��ɷ����ȡ��塢���ԭ�ӽṹ���ɣ���һ�㼴�ɣ���
��
���Թ��ɷ����ȡ��塢���Ӧ���ʵ����ʱ仯���ɣ���һ�㼴�ɣ���
��
��3����֪��Cl2��Br2�Ļ�ѧ�������ƣ�Cl2+H2O��HCl+HClO,����HClOҲ��һ���ᣬ��������������Һ��Ӧ�ķ���ʽΪ��HClO+NaOH��NaClO+ H2O���Էֱ�д��Br2��ˮ��Br2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com