
���� ��1�����ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȣ����з������
��2�����ݻ������ijԪ�ص�����=�û�������������Ԫ�ص��������������з������
��3�����ݻ����������=�û�������ijԪ�ص������¸�Ԫ�ص��������������з������
��� �⣺��1��������̼���⡢������Ԫ�ص�������Ϊ12����1��2��2������14��2����16=3��1��7��4��
��2��30g�����к���Ԫ�ص�����Ϊ30g��$\frac{14��2}{12+16+��14+1��2����2}$��100%=14g��
��3����Ҫ����淋�����Ϊx��x��$\frac{14��2}{��14+1��4����2+32+16��4}��$100%=60g��$\frac{14��2}{12+16+��14+1��2����2}$��100% x=132g��
�ʴ�Ϊ����1��3��1��7��4����2��30g�����к���Ԫ��14g����3��132g�������60g����������Ԫ�������൱��
���� �����ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ���йؼ�����з������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ˮ�����ǵ�����ϢϢ��ء�����ش�������ˮ�йص����⣮
��ˮ�����ǵ�����ϢϢ��ء�����ش�������ˮ�йص����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ѵ������ʵ�ˮ��ɴ����� | B�� | ��ˮ�������� | ||
| C�� | ʹ��������� | D�� | ���Ӷ������������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���͡�
���͡� ���ֱ�������ֲ�ͬ�ĵ��ʷ���A2��B2��������һ���������ܷ�����ѧ��Ӧ���䷴Ӧ����ʾ��ͼ��ͼ��
���ֱ�������ֲ�ͬ�ĵ��ʷ���A2��B2��������һ���������ܷ�����ѧ��Ӧ���䷴Ӧ����ʾ��ͼ��ͼ��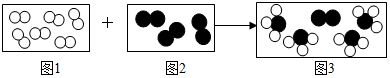
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ǰ���в�����Сѧ���С�����ʳ�á����̣����ΪijУʳ��ij����Ͳ���ʳ�ף�
��ǰ���в�����Сѧ���С�����ʳ�á����̣����ΪijУʳ��ij����Ͳ���ʳ�ף�| ��ʳ | ��� | �ز� |
| �� | ����ţ�� | �����ܲ������ƹ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com