
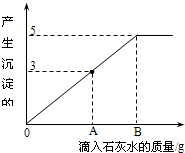
 ��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺
��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺| 106 |
| 100 |
| X |
| 5g |
| 100 |
| 80 |
| 5g |
| y |


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
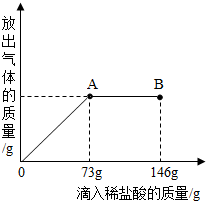 ��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺
��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
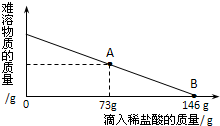 ��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��
��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
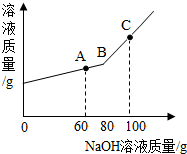 ��2010?���ϣ���һ�ձ���ʢ��һ������������ͭ�������м���������ϡ���ᣬ��ǡ����ȫ��Ӧ����һ���¶��µõ�40gCuSO4�IJ�������Һ����������Һ����ε���������������Ϊ10%��NaOH��Һ����Һ�������������NaOH��Һ��������������ϵ������ͼ��ʾ�����������ش��������⣺
��2010?���ϣ���һ�ձ���ʢ��һ������������ͭ�������м���������ϡ���ᣬ��ǡ����ȫ��Ӧ����һ���¶��µõ�40gCuSO4�IJ�������Һ����������Һ����ε���������������Ϊ10%��NaOH��Һ����Һ�������������NaOH��Һ��������������ϵ������ͼ��ʾ�����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
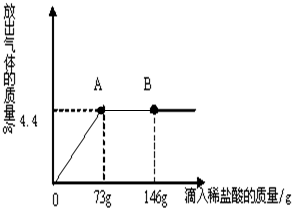 С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�
С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
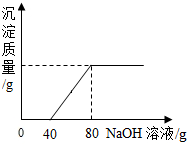
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com