
 ��ͼ�ǡ������������Ƽ���Ʒ��ǩͼ�����ܸ��ݱ�ǩ��Ϣ����������⣺
��ͼ�ǡ������������Ƽ���Ʒ��ǩͼ�����ܸ��ݱ�ǩ��Ϣ����������⣺���� ��1������̼��ƵĻ�ѧʽ��������Է����������ڸ�Ԫ�����ԭ�������ĺͼ������̼��Ƶ���Է�����������Ԫ�ض���̼������ˣ���̼��Ƶ���������Ԫ����̼����е����������������Ԫ�ص�������
��2���������������غ㣬��������ɵĶ�����̼���������ٸ��ݻ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ�����������Ӧ��HCl���������ٸ�����������������ʽ���������ϡ���������ʵ�����������
���ݷ���ʽ�Ļ����Լ����4Ƭ��Ƭ��̼��Ƶ����������������һƬ��Ƭ��̼��Ƶ������������ǩ���Ƚϼ��ɣ�
��� �⣺��1��̼��ƵĻ�ѧʽΪ��CaCO3����̼��Ƶ���Է�������Ϊ��40+12+16��3=100��
̼��ƣ�CaCO3���и�Ԫ�ص���������Ϊ��$\frac{40}{40+12+16��3}$��100%=40%��
��ÿƬ�����ٺ���Ԫ�ص�����Ϊ1.24g��40%=0.50g��
�ʴ�Ϊ��100��0.50��
��2���⣺�ٸ��������غ㶨�ɿ�֪������CO2������Ϊ��40g+4��2.5g-47.8g=2.2g
����μӷ�Ӧ��CaCO3��HCl�������ֱ�Ϊx��y
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 2.2g
$\frac{100}{x}$=$\frac{73}{y}$=$\frac{44}{2.2g}$
���x=5g��y=3.65g
��ϡ���������ʵ���������Ϊ��$\frac{3.65g}{40g}$��100%=9.13%��
��ÿƬ��CaCO3������Ϊ��$\frac{5g}{4}$=1.25g��1.24g���ʸ�Ƭ��̼��Ƶĺ�����עȷ��
�����ɶ�����̼��������2.2g��ϡ���������ʵ���������Ϊ9.13%��ͨ�����㺬����ע��ʵ��
���� ������Ҫ����ѧ�����û�ѧʽ�Լ���ѧ����ʽ���н��м�����������������Ĺؼ��Ǽ������ɶ�����̼��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ���� | ���� | �����Լ��� |
| A | CO2 | CO | ��ȼ |
| B | CO | CO2 | ͨ�����ʯ��ˮ����� |
| C | CuO | C | ����������� |
| D | CaCO3 | CaO | ��ˮ�ܽ�����ϴ�Ӹ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
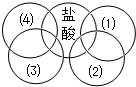 ��ͼ�廷ͼ�е�ÿ�����������л�ѧ�г�����һ�����ʣ������ཻ��ʾ����֮�������Ӧ�����з���������ǣ�������
��ͼ�廷ͼ�е�ÿ�����������л�ѧ�г�����һ�����ʣ������ཻ��ʾ����֮�������Ӧ�����з���������ǣ�������| A�� | ��1��Fe ��2��AgNO3��Һ ��3��Na2CO3��Һ ��4��Ca��OH��2��Һ | |
| B�� | ��1��Cu ��2��AgNO3��Һ ��3��Na2CO3��Һ ��4��Ca��OH��2��Һ | |
| C�� | ��1��Fe ��2��AgNO3��Һ ��3��H2SO4��Һ ��4��Ca��OH��2��Һ | |
| D�� | ��1��CuO ��2��CO ��3��Na2CO3��Һ ��4��Ca��OH��2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ���� | B�� | Ũ���� | C�� | �������ƹ��� | D�� | ����ʯ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ��Ŀ�� | ʵ�鷽�� | |
| A | ̽��ȼ���������� |  |
| B | ̽���������������� |  |
| C | ̽��ʳ�κ��������ˮ���ܽ��� |  |
| D | ʵ������ȡ������̼��ҩƷ�о� |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com