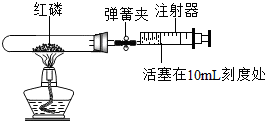
�������ճ������г�����ʳƷ��ij�о�С��ͬѧ���ӻ�ѧ�ĽǶ�����һ���о�����������
��ʵ��̽��һ��ͬѧ�Ƿ��ּ�������ʱ��̫�û���ʲ������һ�ɳ�ζ������ʲôԭ���أ�
�������ϣ�
�ټ������ú����ʲ������⣨H
2S�����壻���������о綾���ܶȱȿ�����������ˮ������ˮ������ԣ�
��ʵ���ҿ�������������ѧʽΪFeS�������ϡ�����ڳ����·�Ӧ����ȡ�������壬ͬʱ��������������
ͬѧ����ʵ������ȡ���ռ���һƿ�������壬�ش��������⣺
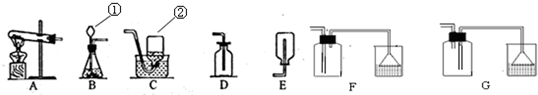
��1��д���б�ŵ�����������
��
����©��
����©��
��
����ƿ
����ƿ
��2��H
2S�����е���Ԫ�صĻ��ϼ�Ϊ
-2
-2
��д��ʵ������ȡ��������Ļ�ѧ����ʽ
FeS+H2SO4=FeSO4+H2S��
FeS+H2SO4=FeSO4+H2S��
���÷�Ӧ����
���ֽ�
���ֽ�
��Ӧ���������Ӧ���ͣ�����ȡ������Ӧѡ�õķ���װ��Ϊ
B
B
������ĸ�����ռ������������ѡ�õ�װ��Ϊ
F
F
������ĸ����������������
��������
��������
��Һ�����գ�
��ʵ��̽�����������ǵijɷ�
��ע�⣺����ʵ������輦�����г�̼�������������ɷֲ������ᷴӦ��Ҳ������ˮ��
��3��С��ͬѧ��֪�����ǵ���Ҫ�ɷ���̼��ƣ���������ȡ������������Ʒ�ھƾ��������գ�һ��ʼ�ŵ�һ���ս���ë��ζ��˵����Ĥ��
������
������
���������պ������DZ߱�ף������ð�ɫ���ʵĻ�ѧ��Ӧ����ʽΪ
����������պ�ĵ���Ͷ������ˮ�У�������ɫ��̪��Һ����Һ��Ϊ
��
��
ɫ��������Ӧ�Ļ�ѧ����ʽΪ
CaO+H2O=Ca��OH��2
CaO+H2O=Ca��OH��2
��
��4��Ϊ�˽�һ���ⶨ������̼��Ƶ�����������С��ͬѧ����������ʵ�飮
����Gװ������Ϊ50.00g������ͼ��װ��10.00g������Ʒ����ϴ�������ﲢ���飩������ƿ�У���������ϡ���ᣬ����ƿ�в��ٲ�������ʱ�����ɼ�C���ӵ���A���������������һ��ʱ������װ��G����Ϊ52.64g��������ԭװ���ڿ�����ʵ���Ӱ�죩��
�ش��������⣺
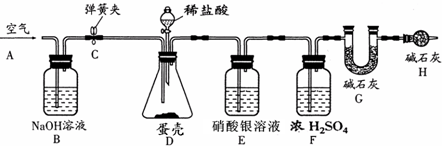
������Ʒǰ��Ӧ
�ӵ���A�������������
�ӵ���A�������������
��װ��D�з�Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��װ��E��������
�����Ȼ�������
�����Ȼ�������
����Ӧ��������ɼ�C���������������Ŀ����
���ɶ�����̼ȫ�����뵽Gװ����
���ɶ�����̼ȫ�����뵽Gװ����
��װ��B��������
���տ����ж�����̼
���տ����ж�����̼
����ȱ��Hװ�ã����õ�̼��Ƶ�����������ƫ
��
��
��С�յ�ʵ���õ�����̼��Ƶ���������Ϊ
60%
60%
��
��5��С��ͬѧ���������һ�ַ�������10.00g������Ʒ����ϴ�������ﲢ���飩�����ձ��Ȼ�����ձ��м�������ϡ���ᣬ���Dz����ܽ⣬�������������ݣ�ʵ����̺Ͳⶨ�����ʵ������������ʾ��

С�µ�ʵ���õ�����̼��Ƶ���������Ϊ
57%
57%
��С����ΪС�µ�ʵ�������һ�����Ե�ȱ�ݻᵼ�²�õ�̼��Ƶĺ���ƫ�ͣ����ȱ����
δϴ��ֱ�Ӻ�ɾͳ�����ɽ��ƫ�Ӷ�ʹ��̼��Ƶĺ���ƫ��
δϴ��ֱ�Ӻ�ɾͳ�����ɽ��ƫ�Ӷ�ʹ��̼��Ƶĺ���ƫ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�




 ij��ѧ��ȤС���ͬѧ�Կ��������������IJⶨʵ�����̽����
ij��ѧ��ȤС���ͬѧ�Կ��������������IJⶨʵ�����̽����