
ʵ������һƿ�����Ƶ�ϡ���ᣨHCl��������ˮ�õ��Ļ����Ϊϡ���ᣩ�����ǩ�IJ���������ͼ��ʾ��
��1�������ϡ��������Ԫ�ص�����������
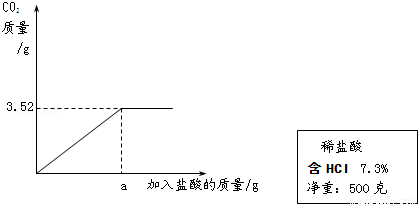
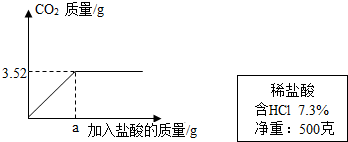
��2��ijʵ��С��������ϡ����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ʵ������ǣ���ȡ10gʯ��ʯ��Ʒ�гɷ�ĩ�������м���7.3%��ϡ���ᣬ����������̼����������������������ϵ��ͼ��ʾ����������Ʒ�е����ʶ��������ᷴӦ������ʯ��ʯ��Ʒ��̼��Ƶ�����������
7.1% 80%
��������
�����������1�����ݻ�ѧʽ���йؼ����Լ���ǩ���ṩ����Ϣ�������
��2������ͼ���֪�÷�Ӧ���ɵĶ�����̼���������ö�����̼����йصķ���ʽ�����������м��㼴�ɣ�
�⣺��1����ϡ��������Ԫ�ص���������Ϊ ��100%=7.1%
��100%=7.1%
��2����ͼ���֪����Ʒ��ȫ��Ӧ�����ɵĶ�����̼������3.52g��
��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.52g
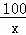 =
=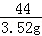
x=8g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ�� ��100%=80%
��100%=80%
�𣺣�1����ϡ��������Ԫ�ص���������Ϊ7.1%����2��ʯ��ʯ��Ʒ��̼��Ƶ�����������80%��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ��㣮
�����������ǶԻ�ѧ�йؼ���Ŀ��飬����Ĺؼ��������йػ�ѧʽ�ͻ�ѧ����ʽ����Ļ���������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ������ʮ��У���꼶3��һģ������ѧ�Ծ����������� ���ͣ�������
ʵ������һƿ�����Ƶ�ϡ���ᣨHCl��������ˮ�õ��Ļ����Ϊϡ���ᣩ�����ǩ�IJ���������ͼ��ʾ��
��1�������ϡ��������Ԫ�ص�����������
��2��ijʵ��С��������ϡ����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ʵ������ǣ���ȡ10gʯ��ʯ��Ʒ�гɷ�ĩ�������м���7.3����ϡ���ᣬ����������̼����������������������ϵ��ͼ��ʾ����������Ʒ�е����ʶ��������ᷴӦ������ʯ��ʯ��Ʒ��̼��Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������һƿ�����Ƶ�ϡ���ᣨHCl��������ˮ�õ��Ļ����Ϊϡ���ᣩ�����ǩ�IJ���������ͼ��ʾ��
��1�������ϡ��������Ԫ�ص�����������
��2��ijʵ��С��������ϡ����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ʵ������ǣ���ȡ10gʯ��ʯ��Ʒ�гɷ�ĩ�������м���7.3����ϡ���ᣬ����������̼����������������������ϵ��ͼ��ʾ����������Ʒ�е����ʶ��������ᷴӦ������ʯ��ʯ��Ʒ��̼��Ƶ�����������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�갲��ʡ������ʮ��У�п���ѧһģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com