
����Cu����һ�ֽ�����������Mg��Fe��Zn�е�һ�֣��γɵķ�ĩ�������ⶨ����ɣ���������ʵ�飺ȡ�÷�ĩ16g�����ձ�����������������Ϊ14%��ϡ����280.0g��4�μ�����ձ��У���ַ�Ӧ���ʣ��Ĺ����������ݼ�¼���£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����ϡ����������g | 70.0 | 70.0 | 70.0 | 70.0 |
| ʣ�����������g | 13.6 | 11.2 | 8.8 | 8.4 |
��1��52.5 % ��2�֣�
��2���ý������ԭ������Ϊ 24 ���Խ���Ϊþ ��3�֣�
��3��16.6% ��3�֣�
���������������1��Cu���ܺ�ϡH2SO4��Ӧ��ʣ���������ΪCu��������
�÷�ĩ��Cu���������� =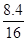 �� 100% = 52.5%
�� 100% = 52.5%
��2����÷�ĩ����һ�ֽ���Ϊ M���ý������ԭ������Ϊx
M + H2SO4 = MSO4 + H2 ��
X 98
2.4 70��14%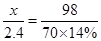
x =" 24"
�ý������ԭ������Ϊ 24 ���Խ���Ϊþ
��3���μӷ�Ӧ��þ������Ϊ7.6g ��������ҺΪ����þ��Һ����������þ��Һ����������������
�� þ��ϡ���ᷴӦ��������þ������Ϊy g ����������������Ϊ z g .
Mg + H2SO4 = MgSO4 + H2 ��
24 120 2
7.2 y z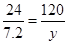
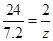
y = 36g z = 0.6g
��3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ���������= 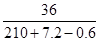 �� 100% = 16.6%
�� 100% = 16.6%
�� ����
���㣺���û�ѧ����ʽ���㡣


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��Ԫ������������Ԫ�أ������ʳ������ȡ���㣬ҽ�����顰���ơ���
��1��ҽ�����顰���ơ���ָ������ �����Ԫ�ء����ʡ���ԭ�ӡ���
��2��̼�����̼Ԫ�صĻ��ϼ�Ϊ�� ����
��3��Ϊ�ⶨ�ò��Ƽ���̼��Ƶ������������ֳ�ȡ15�˵���Ʒ�����ձ�������м���������ϡ�����ַ�Ӧ�������ɷֲ�����Ԫ�ء�������ˮҲ����ϡ���ᷴӦ������������Ϊ4.4g��
���ж���ȡ��Ʒ����ȫ��Ӧ������Ϊ�� ����
�ڸò��Ƽ���Ʒ��̼��Ƶ���������Ϊ���٣������ü����������ʾ������������һλС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ��������������������
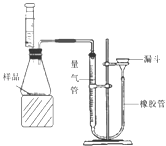
��1���������ӳ��û�ͭ�Ƴɽ�������ġ���Ԫ������ƭ���ǣ����м����д�������� ����
| A������ɫ | B������ | C������������Һ | D�������� |
| | �Ͻ������/g | ϡ��������/mL | ��������������/g |
| ��1�� | 2 | 15 | 0.04 |
| ��2�� | 2 | 20 | 0.04 |
| ��3�� | 4 | 15 | 0.04 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ҵ�ϣ���������ʯ��ʯ��CaCO3�����Ƶ���ʯ�ң�CaO���Ͷ�����̼������100��ʯ��ʯ������һ��ʱ���������Ϊ78�֡�
��1���Ƶö�����̼���ٶ֣�
��2��ʯ��ʯ��CaCO3����������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ڵ������������л����һ�����ĺ�ͭ���ϣ��ݱ�������ͨ����������ȵ������£�ͭ��ϡ���ᷴӦת��Ϊ����ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ��2Cu+2H2SO4+O2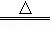 2CuSO4+2H2O������һ�������ʵ���������Ϊ9.8%��ϡ����ǡ�ô���2000g��ͭ3.2%�ķ��ϣ������������ʲ������ᷴӦ�Ҳ�����ˮ������Ӧ����������ͭ��Һ�����ʵ�����������
2CuSO4+2H2O������һ�������ʵ���������Ϊ9.8%��ϡ����ǡ�ô���2000g��ͭ3.2%�ķ��ϣ������������ʲ������ᷴӦ�Ҳ�����ˮ������Ӧ����������ͭ��Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2����֪SiO2�Ȳ�����ˮҲ�������ᷴӦ������ȡ8gʯ��ʯ��Ʒ��������50gϡ������ȫ��Ӧ�Ƶ������ڵ�����������Ϊ55��36g��
�Իش��������⣺
��1����Ӧ�������ڵ������� ��
��2����Ӧ����������̼������Ϊ ��
��3����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10(PO4)6(OH)2]��ʽ���ڣ�����Է�������Ϊ1004��ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֡�����ϸ�Ķ���ش��������⣺
��1����װ��ǩ��֬����3.3g����ָ100mLţ���к�֬������������3.3g����ôһ��ţ�̺������� g��������0.01g����
��2�����ǻ�������и�Ԫ�ص���������������Ϊ0.1%����
��3��������ÿ��������Ҫ0.6g�ƣ�����Щ����90%����ţ�̣���һ����ÿ������Ҫ�ȶ��ٺ�ţ�̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��ѧ��ȤС��Ϊ�˲ⶨijϡ�������������������ȡ100gϡ�������ձ��У����ձ�����ε�����������Ϊ10%������������Һ����Ӧ������������Һ�����ȱ仯��ͼ��
��1������������������Һ������Ϊa gʱ��������Һ�е������� ���ѧʽ����
����������������Һ������Ϊb gʱ����������Һ�еμӷ�̪��Һ����Һ�� ɫ��
��2��ϡ������������������Ƕ��٣�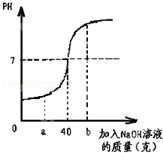
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ����ȣ�ݣ����������ʱ����ľ�Ǵ���C5H12O5���������ǣ�
��1��ľ�Ǵ�����Է�������Ϊ ��
��2��һ��ľ�Ǵ��������� ��ԭ�ӣ�������̼���⡢��ԭ�ӵĸ�����Ϊ ��
��3��ľ�Ǵ���̼Ԫ�ص���������Ϊ �����������0.1%����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com