
 п�����彡���������Ԫ�أ�ȱп������ɷ����ϰ�������ʳ�Ȳ�֢��ʹ�������߹����½������۵���������п�ڷ�Һ��пȱ��֢���нϺõ���Ч����ͼ��ijƷ�Ƶı�ǩ���ش����⣺
п�����彡���������Ԫ�أ�ȱп������ɷ����ϰ�������ʳ�Ȳ�֢��ʹ�������߹����½������۵���������п�ڷ�Һ��пȱ��֢���нϺõ���Ч����ͼ��ijƷ�Ƶı�ǩ���ش����⣺| 65 |
| 455 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ư�ɫ�Ļ��� |
| B�������Ϸ������һ�����ձ�ʱ�ܷ����ձ��ڱ�����ˮ�� |
| C���������洦�ۻ����Һ�� |
| D������ȼ��ʱŨ�̹��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڼ���վ���������ƶ��绰 |
| B����¥��������ʱ�������Ŵ�ͨ�� |
| C�����������Ż𣬿���Һ̬������̼�˻� |
| D���������õ�������ǰ�Ƚ��л��ʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��K2CO3��NH4NO3��Ca��OH��2 |
| B��NH4NO3��H2SO4��BaCl2 |
| C��AlCl3��NaNO3��CaCl2 |
| D��Fe2��SO4��3��H2SO4��Ca��NO3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Cr2O3 |
| B��Cr2��SO4��3 |
| C��CrSO3 |
| D��Cr2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
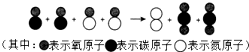 ����β���к���һ����̼��һ�������������ø�Ч������һ�������½�����ת��Ϊ�����壬��Ӧ���۹���ʾ��ͼ��ͼ��ʾ����ֱ�����ʵ���ɣ����ʣ�����ӣ��Ĺ��ɼ���仯���棬д��������õĸ�����Ϣ����һ����
����β���к���һ����̼��һ�������������ø�Ч������һ�������½�����ת��Ϊ�����壬��Ӧ���۹���ʾ��ͼ��ͼ��ʾ����ֱ�����ʵ���ɣ����ʣ�����ӣ��Ĺ��ɼ���仯���棬д��������õĸ�����Ϣ����һ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com