
 Ä³Ń§Š£æĘѧŠĖȤŠ”×éĪŖĮĖĢ½¾æŹµŃéŹŅÖŠ¾ĆÖƵÄNaOHµÄ±äÖŹ³Ģ¶Č£¬¾ßĢåČēĶ¼£ŗ
Ä³Ń§Š£æĘѧŠĖȤŠ”×éĪŖĮĖĢ½¾æŹµŃéŹŅÖŠ¾ĆÖƵÄNaOHµÄ±äÖŹ³Ģ¶Č£¬¾ßĢåČēĶ¼£ŗ·ÖĪö £Ø1£©øł¾ŻĒāŃõ»ÆÄĘÄÜŗĶæÕĘųÖŠµÄ¶žŃõ»ÆĢ¼·“Ӧɜ³ÉĢ¼ĖįÄĘŗĶĖ®£¬ÄÜŗĶĻ”ŃĪĖį·“Ӧɜ³ÉĀČ»ÆÄĘŗĶĖ®£»Ģ¼ĖįÄĘŗĶĻ”ŃĪĖį·“Ӧɜ³ÉĀČ»ÆÄĘ”¢Ė®ŗĶ¶žŃõ»ÆĢ¼£¬ĄūÓƶžŃõ»ÆĢ¼µÄÖŹĮæĒó³öĢ¼ĖįÄʵÄÖŹĮ漓æÉ£»
£Ø2£©øł¾ŻĒāŃõ»ÆÄʵÄÖŹĮæĒó³öŹµŃé¹ż³ĢÖŠÓėNaOH·“Ó¦ĖłÓƵÄŃĪĖįČÜÖŹµÄÖŹĮ漓æÉ£»
£Ø3£©øł¾ŻĢ¼ĖįÄʵÄÖŹĮæĒó³ö±äÖŹµÄĒāŃõ»ÆÄʵÄÖŹĮ棬½ų¶ųĒó³öѳʷ֊NaOHµÄ±äÖŹ³Ģ¶Č¼“æÉ£®
½ā“š ½ā£ŗ£Ø1£©ÉčĢ¼ĖįÄʵÄÖŹĮæĪŖx£¬
ÓÉĶ¼ÖŠŠÅĻ¢æÉÖŖ£¬²śÉś¶žŃõ»ÆĢ¼µÄÖŹĮæŹĒ2.2g£¬
Na2CO3+2HClØT2NaCl+H2O+CO2”ü£¬
106 44
x 2.2g
$\frac{106}{x}=\frac{44}{2.2g}$
x=5.3g
£Ø2£©ÉčŗĶĒāŃõ»ÆÄĘ·“Ó¦µÄĀČ»ÆĒāÖŹĮæĪŖy£¬
ŗĶĀČ»ÆĒā·“Ó¦µÄĒāŃõ»ÆÄĘÖŹĮæĪŖ£ŗ13.3g-5.3g=8g£¬
NaOH+HCl=NaCl+H2O£¬
40 36.5
8g y
$\frac{40}{8g}=\frac{36.5}{y}$
y=7.3g£¬
£Ø3£©Éč±äÖŹµÄĒāŃõ»ÆÄĘÖŹĮæĪŖz£¬
2NaOH+CO2ØTNa2CO3+H2O£¬
80 106
z 5.3g
$\frac{80}{z}=\frac{106}{5.3g}$
z=4g
“ĖNaOHµÄ±äÖŹ³Ģ¶ČĪŖ£ŗ$\frac{4g}{13.3g-5.3g+4g}$”Į100%=33.3%£¬
“š£ŗ£Ø1£©øĆѳʷ֊Na2CO3µÄÖŹĮæŹĒ5.3g£»
£Ø2£©ŹµŃé¹ż³ĢÖŠÓėNaOH·“Ó¦ĖłÓƵÄŃĪĖįČÜÖŹµÄÖŹĮæĪŖ7.3g£»
£Ø3£©“ĖNaOHµÄ±äÖŹ³Ģ¶ČĪŖ33.3%£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éѧɜŌĖÓĆ¼ŁÉč·ØŗĶ»Æѧ·½³ĢŹ½½ųŠŠ¼ĘĖćŗĶĶʶĻµÄÄÜĮ¦£¬Ķ¬Ź±æ¼²éĮĖ·ÖĪöĶ¼ÖŠŹż¾ŻµÄÄÜĮ¦£¬¼ĘĖ揱ŅŖ×¢Ņā¹ę·¶ŠŌŗĶ×¼Č·ŠŌ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ķ¬Ń§ĆĒŅŌøĒ·æ×ӵķ½Ź½×ܽį³öĮĖČēĻĀĪļÖŹ¼äµÄ¹ŲĻµ£®A”¢B”¢C¾łĪŖ“æ¾»Īļ£¬ĒŅÉĻ”¢ĻĀĻąĮŚµÄĪļÖŹÖ®¼ä¾łæÉ·¢Éś·“Ó¦£®»Ų“šĻĀĮŠĪŹĢā£ŗ
Ķ¬Ń§ĆĒŅŌøĒ·æ×ӵķ½Ź½×ܽį³öĮĖČēĻĀĪļÖŹ¼äµÄ¹ŲĻµ£®A”¢B”¢C¾łĪŖ“æ¾»Īļ£¬ĒŅÉĻ”¢ĻĀĻąĮŚµÄĪļÖŹÖ®¼ä¾łæÉ·¢Éś·“Ó¦£®»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| Ń”Ļī | A | B | C | D |
| ŹµŃéÄæµÄ | ³żČ„ĒāŃõ»ÆÄĘÖŠ ÉŁĮæµÄĢ¼ĖįÄĘ | ĒåĻ“ĢśÖĘĘ· ±ķĆęµÄĢśŠā | ¼ų±šĀČ»ÆÄĘ ČÜŅŗŗĶĻ”ŃĪĖį | ¼ģŃéŅ»Ńõ»ÆĢ¼ÖŠŹĒ·ń»ģÓŠÉŁĮ涞Ńõ»ÆĢ¼ |
| ·½°ø1 | ¼ÓĖ®Čܽā | ¼ÓŹŹĮæĻ”ŃĪĖį | ¼ÓŠæĮ£ | µćČ¼ |
| ·½°ø2 | ¼ÓĻ”ĮņĖį | ÓĆĖ®Ļ“µÓ | ¼ÓŹÆČļČÜŅŗ | ĶØČė³ĪĒåŹÆ»ŅĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
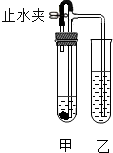 ijĶ¬Ń§ÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃé£ØĶ¼ÖŠĢś¼ÜĢصČŅĒĘ÷¾łŅŃĀŌČ„£©£®ŌŚ¼×ŹŌ¹ÜÖŠ¼ÓČėŹŌ¼Įŗó£¬Čū½ōĻšĘ¤Čū£¬Į¢¼““ņæŖÖ¹Ė®¼Š£¬ŅŅŹŌ¹ÜÖŠÓŠĘųÅŻĆ°³ö£»Ņ»¶ĪŹ±¼äŗó¹Ų±ÕÖ¹Ė®¼Š£¬ŅŅŹŌ¹ÜÖŠŅŗĆęÉĻÉż£¬ČÜŅŗÓɳĪĒå±ä»ė×Ē£®·ūŗĻÉĻŹöŹµŃéĻÖĻóµÄ¼×”¢ŅŅŹŌ¹ÜÖŠÓ¦¼ÓČėµÄŹŌ¼ĮŹĒ£Ø””””£©
ijĶ¬Ń§ÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃé£ØĶ¼ÖŠĢś¼ÜĢصČŅĒĘ÷¾łŅŃĀŌČ„£©£®ŌŚ¼×ŹŌ¹ÜÖŠ¼ÓČėŹŌ¼Įŗó£¬Čū½ōĻšĘ¤Čū£¬Į¢¼““ņæŖÖ¹Ė®¼Š£¬ŅŅŹŌ¹ÜÖŠÓŠĘųÅŻĆ°³ö£»Ņ»¶ĪŹ±¼äŗó¹Ų±ÕÖ¹Ė®¼Š£¬ŅŅŹŌ¹ÜÖŠŅŗĆęÉĻÉż£¬ČÜŅŗÓɳĪĒå±ä»ė×Ē£®·ūŗĻÉĻŹöŹµŃéĻÖĻóµÄ¼×”¢ŅŅŹŌ¹ÜÖŠÓ¦¼ÓČėµÄŹŌ¼ĮŹĒ£Ø””””£©| A | B | C | D | |
| ¼× | Zn”¢Ļ”H2SO4 | Cu”¢Ļ”H2SO4 | CaCO3”¢Ļ”HCl | Na2CO3”¢Ļ”H2SO4 |
| ŅŅ | BaCl2 | Ba£ØOH£©2 | KNO3 | NaCl |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³ĪĒåŹÆ»ŅĖ® | B£® | ×ĻÉ«ŹÆČļŹŌŅŗ | C£® | “ų»šŠĒµÄľĢõ | D£® | µŖĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | 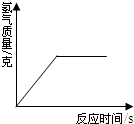 | B£® |  | C£® |  | D£® |  |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com