
ĀĮ”¢Ģś”¢ĶŹĒĪŅĆĒÉś»īÖŠŹ¹ÓĆ±Č½Ļ¹ć·ŗµÄ½šŹō”£
(1)ŅŌÉĻÓĆĘ·ÖŠ£¬Ö÷ŅŖĄūÓĆ½šŹōµ¼µēŠŌµÄŹĒ (ĢīŠņŗÅ)”£
(2)ĀĮÖĘĘ·±ķĆęµÄĪŪ×Õ²»ŅĖÓĆøÖĖæĒņ²ĮĻ“£¬ŅŌĆāĘĘ»µ±ķĆęµÄ ”£
(3)ĒŠ¹żŹß²ĖµÄ²Ėµ¶£¬øéÖĆŌŚÕč°åÉĻŅ»¶ĪŹ±¼äŗóÉśŠāĮĖ”£ĢśŌŚæÕĘųÖŠŠāŹ“£¬Źµ¼ŹÉĻŹĒĢśøś µČĪļÖŹ·¢Éś»Æѧ·“Ó¦µÄ½į¹ū”£
(4)ŠāŹ“øÖĢś»ŲŹÕÖŲŠĀŅ±Į¶ŹĒ±£»¤½šŹō׏Ō“µÄŅ»ĢõÓŠŠ§Ķ¾¾¶”£Š“³öÓĆŅ»Ńõ»ÆĢ¼ŌŚøßĪĀĢõ¼žĻĀ»¹ŌŠāŹ“øÖĢśµÄ»Æѧ·½³ĢŹ½ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĀĮ”¢Ģś”¢ĶŹĒČĖĄą¹ć·ŗŹ¹ÓƵÄČżÖÖ½šŹō£¬ÓėĪŅĆĒÉś»īĻ¢Ļ¢Ļą¹Ų£®
£Ø1£©ŌŚæÕĘųÖŠ”” ÖĘĘ·£ØĢī”°ĀĮ”±»ņ”°Ģś”±£©øüÄĶøÆŹ“£®
£Ø2£©ČĖĆĒ“óĮæŹ¹ÓƵďĒŗĻ½š¶ų²»ŹĒ“潚Źō£¬ÕāŹĒŅņĪŖŗĻ½š¾ßÓŠøü¶ąÓÅĮ¼ŠŌÄÜ£¬ĄżČēøֱȓæĢśÓ²¶Č””””£ØĢī”°“ó”±»ņ”°Š””±£©£®
£Ø3£©ĮņĖįŗĶŃĪĖį¶¼ÄܳżĢśŠā£¬Š“³öŃĪĖįÓėĢśŠāÖ÷ŅŖ³É·Ö·“Ó¦µÄ»Æѧ·½³ĢŹ½”” £®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Į¶ĢśµÄŌĄķŹĒĄūÓĆŅ»Ńõ»ÆĢ¼ÓėŃõ»ÆĢś·“Ó¦£¬Ä³Ķ¬Ń§ĄūÓĆøĆŌĄķÉč¼ĘĮĖŅ»øöŹµŃ飬ŹµŃé×°ÖĆ¼ūĻĀĶ¼£ŗ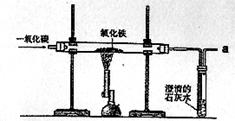
¶ŌŹµŃé½ųŠŠ·ÖĪö²¢»Ų“š£ŗ
£Ø1£©ŹµŃéÖŠ²£Į§¹ÜĄļŃõ»ÆĢś·ŪÄ©µÄŃÕÉ«±ä»ÆŹĒ ”£¹Ū²ģµ½³ĪĒåŹÆ»ŅĖ®µÄĻÖĻóŹĒ £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£Ø2£©ŹµŃéĒ°”°ĶØČėCO”±Óė”°æŖŹ¼¼ÓČČ”±µÄĖ³ŠņŹĒ ”£
£Ø3£©“Ó»·±£½Ē¶Č·ÖĪö£¬øĆĮ÷³ĢÉč¼ĘÖŠµÄŅ»“¦Ć÷ĻŌ²»×ćŹĒ”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øÖĢśŹĒŹ¹ÓĆ×ī¶ąµÄ½šŹō²ÄĮĻ”£
£Ø1£©ĢśÖĘĘ·ŌŚŅ»¶ØĢõ¼žĻĀÄÜÉśŠā£¬ŹčĖɵÄĢśŠā²»ÄÜ×č°Ąļ²ćµÄĢś¼ĢŠųÓė ·“Ó¦£¬Ņņ“ĖĢśÖĘĘ·æÉŅŌ½ųŅ»²½ŠāŹ“”£·ĄÖ¹×ŌŠŠ³µĮ“ĢõÉśŠāµÄŗĻĄķ“ėŹ©ŹĒ ”£ÓĆŃĪĖį³żĢśŠāµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓĆŅ»Ńõ»ÆĢ¼ŗĶ³ąĢśæóĮ¶ĢśµÄ»Æѧ·½³ĢŹ½ĪŖ ”£»ŲŹÕĄūÓĆ·Ļ¾É½šŹōÖĘĘ·µÄÄæµÄÖ®Ņ»ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŹµŃ銔×éÄ£ÄāøßĀÆĮ¶ĢśµÄ»Æѧ·“Ó¦ŌĄķ½ųŠŠŹµŃ飬Ęä×°ÖĆČēĻĀĶ¼ĖłŹ¾”£
£Ø1£©A“¦·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø2£©BÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ”””””””””””””””””””£
C“¦µćČ¼¾Ę¾«µĘµÄÄæµÄŹĒ”””””””””””””””””””””””£
£Ø3£©ŹµŃé½įŹųŗ󣬊”Ąī擵½ŗģÉ«¹ĢĢåČ«²æ±äŗŚĮĖ£¬ČĻĪŖ²śĪļ¾ĶŹĒµ„ÖŹĢś£¬µ«Š”ĶõĢį³öĮĖÖŹŅÉ£ŗ»¹ÓŠæÉÄÜÉś³ÉĘäĖüĪļÖŹ”£ĪŖĮĖ½ųŅ»²½ŃéÖ¤Ęä¹ĢĢå³É·Ö£¬Š”Ķõ²éµ½ĻĀĮŠ×ŹĮĻ£ŗ¢ŁĢśµÄŃõ»ÆĪļÓŠŃõ»ÆĢś”¢Ńõ»ÆŃĒĢś”¢ĖÄŃõ»ÆČżĢś£ØøĆ»ÆŗĻĪļÖŠĢśÓŠ£«2”¢£«3Į½ÖÖ»ÆŗĻ¼Ū£©£¬ĘäÖŠŃõ»ÆĢśĪŖŗģ×ŲÉ«£¬ĘäÓą¶¼ĪŖŗŚÉ«£»²¢ĒŅÖ»ÓŠĖÄŃõ»ÆČżĢśÄܱ»“ÅĢśĪüŅż”£¢ŚĢśµÄŃõ»ÆĪļ¶¼ÄÜÓėĖį·¢Éś·“Ó¦¶ųČܽā”£¢Ū»ĘÉ«µÄČÜŅŗ£ŗŗ¬ÓŠFe3+µÄČÜŅŗ£ØČēĀČ»ÆĢś”¢ĮņĖįĢś”¢ĻõĖįĢś£©
øł¾ŻÉĻŹöŠÅĻ¢£¬Š”ĶõÓÖÉč¼ĘĮĖĮ½øöŹµŃ锣
[ŹµŃé1]
½«ŗŚÉ«¹ĢĢåŃŠÄ„ŗó£¬Č”ÉŁĮæ¼ÓČė””””””””ČÜŅŗÖŠ£¬¹Ū²ģµ½ÓŠĘųÅŻ³öĻÖ£¬ČÜŅŗĪ“±ä»ĘÉ«£¬¾Ż“ĖµĆ³öµÄ½įĀŪŹĒ”””””””””””””””””””””””£
[ŹµŃé2]
ŌŁÓĆ“ÅĢśĪüŅżÓąĻĀŗŚÉ«¹ĢĢ壬ŹµŃéŗó¼“æɶŌŗŚÉ«¹ĢĢå³É·Ö½ųŠŠÅŠ¶Ļ£ŗČē¹ū”””””””””” £»Čē¹ū ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ŹĒ¼×Ķ¬Ń§Éč¼ĘµÄŅ»Ńõ»ÆĢ¼ÓėŃõ»ÆĢśµÄ·“Ó¦ŹµŃé×°ÖĆ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹµŃ鏱ӦĻȵćČ¼________(Ģī”°A”±»ņ”°B”±)“¦µÄ¾Ę¾«µĘ”£
£Ø2£©·“Ó¦ÖŠÓ²ÖŹ²£Į§¹ÜÄŚµÄĻÖĻóĪŖ £»·¢Éś·“Ó¦µÄ·½³ĢŹ½ĪŖ ”£
£Ø3£©¾Ę¾«µĘBµÄ×÷ÓĆŹĒ ”£
£Ø4£©ŅŅĶ¬Ń§ČĻĪŖ¼×Éč¼ĘµÄ×°ÖĆÖŠĪ²Ęų“¦Ąķ»¹æÉŅŌÓĆĘäĖū·½·Ø£¬ĒėÄćĪŖŅŅĶ¬Ń§Éč¼ĘŅ»ÖÖŗĻĄķµÄĪ²Ęų“¦Ąķ·½·Ø£ŗ________________________________________”£
£Ø5£©±ūĶ¬Ń§ÖŹŅÉCOÄÜ·ńŹ¹ŹÆ»ŅĖ®±ä»ė×Ē£¬Ņņ“ĖĘäÉč¼ĘŌŚCOĶØČėFe2O3Ö®Ē°Ó¦ĻČĶعż³ĪĒåŹÆ»ŅĖ®ŅŌÅųżCOÓė³ĪĒåŹÆ»ŅĖ®·“Ó¦£¬ŹŌ¶Ō“Ė×ö³öĘĄ¼Ū£¬ÄćČĻĪŖ±ūµÄÉč¼Ę________(Ģī”°±ŲŅŖ”±»ņ”°²»±ŲŅŖ”±)£¬ĄķÓÉŹĒ ___________________________”£
£Ø6£©¶”Ķ¬Ń§ĪŖ¼ģŃé·“Ó¦ÓŠĢśÉś³É½ųŠŠČēĻĀŹµŃé£ŗ½«·“Ó¦ŗóµÄ¹ĢĢåȔɣĮæ·ÅČėĻ”ŃĪĖįÖŠ¹Ū²ģµ½ÓŠĘųÅŻĆ°³ö”£ĒėŠ“³öøĆ·“Ó¦µÄ·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijĶ¬Ń§ÓĆĻąĶ¬µÄĢś¶¤Ģ½¾æĢśÉśŠāÓėÄÄŠ©ŅņĖŲÓŠ¹Ų£¬Éč¼ĘµÄŹµŃéČēĶ¼”£¾¹żŅ»ÖܹŪ²ģ£ŗŹŌ¹ÜAŗĶCÖŠµÄĢś¶¤ĪŽĆ÷ĻŌ±ä»Æ£¬ŹŌ¹ÜBÖŠµÄĢś¶¤Ć÷ĻŌŠāŹ“”£
£Ø1£©ĶعżĢ½¾æ·¢ĻÖ£ŗĢśÉśŠāŹĒĢśÓė ¹²Ķ¬“ęŌŚŹ±·¢Éś»Æѧ·“Ó¦µÄ¹ż³Ģ”£
£Ø2£©AÖŠ¼ÓČėµÄÕōĮóĖ®ŅŖŹĀĻČÖó·Š£¬ĘäÄæµÄŹĒ £»
£Ø3£©ĶعżĢ½¾æ£¬ĒėÄ抓³ö·ĄÖ¹ĢśÖĘĘ·ÉśŠāµÄŅ»ÖÖ“ėŹ© ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø11·Ö£©½šŹōŌŚÉś²śÉś»īÖŠÓ¦ÓĆ¹ć·ŗ”£
£Ø1£©ĻĀĶ¼ŹĒĀĮµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ ”£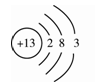
A£®ĀĮŌ×ÓµÄÖŹ×ÓŹżĪŖ13 B£®»ÆŗĻĪļÖŠĀĮŌŖĖŲĶس£ĻŌ+3¼Ū
C£®ĀĮŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ D£®ĀĮæÉ×÷µ¼ĻߏĒÓÉÓŚĖü¾ßÓŠĮ¼ŗƵĵ¼µēŠŌ
£Ø2£©21ŹĄ¼ĶŹĒīѵďĄ¼Ķ”£ĻĀĆęŹĒĄūÓĆīŃ°×·Ū(TiO2)Éś²śŗ£ĆąīŃ(Ti)µÄŅ»ÖÖ¹¤ŅÕĮ÷³Ģ£ŗ 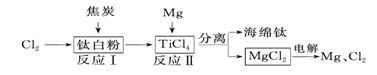
¢Ł·“Ó¦¢ńŌŚ800”«900 ”ęµÄĢõ¼žĻĀ½ųŠŠ£¬»¹Éś³ÉŅ»ÖÖæÉČ¼ŠŌĪŽÉ«ĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___ __£»
¢Ś·“Ó¦IIæÉ»ńµĆŗ£½õīŃ£¬»Æѧ·½³ĢŹ½±ķŹ¾ĪŖTiCl2+2Mg øßĪĀTi+2MgCl2”£øĆ·“Ó¦ŹōÓŚ
(ĢīŠņŗÅ)”£
A£®»ÆŗĻ·“Ó¦ B£®·Ö½ā·“Ó¦ C£®ÖĆ»»·“Ó¦ D£®ø“·Ö½ā·“Ó¦
¢ŪøĆ¹¤ŅÕĮ÷³ĢÖŠ£¬æÉŃ»·ĄūÓƵÄĮ½ÖÖĪļÖŹŹĒ ”¢ ”£
£Ø3£©ĻÖÓŠĀĮ”¢Ģś”¢Ķ”¢ŅųĖÄÖÖ½šŹōĻą»„×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾”£ ŹŌĶĘĄķŅŅŹĒ £»Š“³ö¼×”ś±ū·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£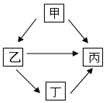
£Ø4£©ĻņŅ»¶ØÖŹĮæAgNO3ŗĶCu(NO3)2µÄ»ģŗĻČÜŅŗÖŠ¼ÓČėZn£¬ČÜŅŗÖŹĮæÓė¼ÓČėZnµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ ”£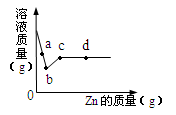
A£®aµćČÜŅŗÖŠµÄČÜÖŹÓŠ4ÖÖ
B£®cµćČÜŅŗÖŠČÜÖŹĪŖZn(NO3)2
C£®ČōČ”b”«c¶ĪČÜŅŗ£¬µĪ¼ÓĻ”ŃĪĖį£¬ÓŠ°×É«³Įµķ
D£®Č”dµćµÄ¹ĢĢ壬¼ÓČėĻ”ŃĪĖį£¬ÓŠĘųÅŻ²śÉś
£Ø5£©ĻąĶ¬ÖŹĮæµÄŠæ”¢Ģś·Ö±šÓėĻ”ŃĪĖį×÷ÓĆ£¬²śÉśH2µÄÖŹĮæm(H2)Óė¼ÓČėĻ”ŃĪĖįµÄĢå»żV(Ļ”ŃĪĖį)¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬ĘäÖŠÕżČ·µÄŹĒ ”£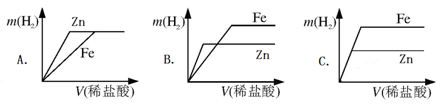
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
Š”Ć÷·¢ĻÖŌĀ±żŗŠĄļµÄĶŃŃõ¼Į²æ·Ö³ŹŗģŗÖÉ«£¬²éŌÄ׏ĮĻµĆÖŖĶŃŃõ¼ĮÖŠŗ¬ÓŠĢś·ŪŗĶ»īŠŌĢ攣Ėū²ĀĻėÕā°üĶŃŃõ¼ĮÖŠæÉÄÜŗ¬ÓŠ£ŗ¢ŁCaŗĶC£»¢ŚFe2O3ŗĶC£»¢ŪFe3O4ŗĶC£»¢ÜFe3O4”¢CŗĶFe£»¢ŻFe2O3”¢CŗĶFe”£ĘäÖŠ²ĀĻėŗĻĄķµÄŹĒ( )
| A£®¢Ł¢Ū¢Ż | B£®¢Ś¢Ü¢Ż | C£®¢Ś¢Ż | D£®¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com