
 ��1�����к�̼Ԫ�ص������У������л������
��1�����к�̼Ԫ�ص������У������л������

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
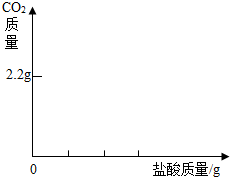 ijѧ���ú���̼�������ʵ��ռ���Ʒ�����ᷢ����Ӧ��ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g 7.3%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����������ȫ���ݳ�����
ijѧ���ú���̼�������ʵ��ռ���Ʒ�����ᷢ����Ӧ��ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g 7.3%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����������ȫ���ݳ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������ͼ��ʾװ�ã�β�����������ԣ�����ȡһ����̼�������Բⶨijͭ����Ʒ������CuO��ĩ���н���ͭ�ĺ�����
��������ͼ��ʾװ�ã�β�����������ԣ�����ȡһ����̼�������Բⶨijͭ����Ʒ������CuO��ĩ���н���ͭ�ĺ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
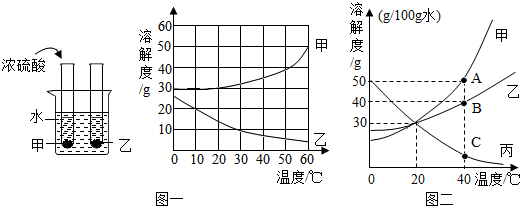
 ��ͼ��ʾ�Ǽס����������ʵ��ܽ�����ߣ���ش��������⣺
��ͼ��ʾ�Ǽס����������ʵ��ܽ�����ߣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ļʽ����� |
| B����ɫ��ͼ�� |
| C����ɫ���й��� |
| D��ҹ������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com