
 ÓŠŠ©µŲ·½ČĖĆĒ³£ÓĆĆŗĀÆĄ“ÉÕĖ®”¢Č”ÅÆµČ£¬ĘäČ¼ĮĻÓŠµÄŹĒÖĘ×÷³ÉŠĶµÄ”°·äĪŃĆŗ”±£®
ÓŠŠ©µŲ·½ČĖĆĒ³£ÓĆĆŗĀÆĄ“ÉÕĖ®”¢Č”ÅÆµČ£¬ĘäČ¼ĮĻÓŠµÄŹĒÖĘ×÷³ÉŠĶµÄ”°·äĪŃĆŗ”±£®
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ038
(2007
£¬ŌĘÄĻ)ÓŠŠ©µŲ·½ČĖĆĒ³£ÓĆĆŗĀÆĄ“ÉÕĖ®”¢Č”ÅÆµČ£¬ĘäČ¼ĮĻÓŠµÄŹĒÖĘ×÷³ÉŠĶµÄ”°·äĪŃĆŗ”±£®(1)
”°·äĪŃĆŗ”±¼Ó¹¤³É¶ąæ׊ĪדµÄŌŅņŹĒ____________________________£®(2)
ÖĘ×÷”°·äĪŃĆŗ”±Ź±³£¼ÓČėÉśŹÆ»Ņ£¬ÓÉ“ĖŌŚĆŗĀÆÖŠæÉ·¢ÉśČēĻĀ»Æѧ·“Ó¦£ŗ

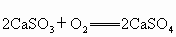
ÉĻŹö·“Ó¦ŹōÓŚ
_____________·“Ó¦(Ģī»ł±¾·“Ó¦ĄąŠĶ)£»ÖĘ×÷”°·äĪŃĆŗ”±Ź±¼ÓČėÉśŹÆ»ŅµÄ×÷ÓĆŹĒ__________________________________________£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ÓŠŠ©µŲ·½ČĖĆĒ³£ÓĆĆŗĀÆĄ“ÉÕĖ®”¢Č”ÅÆµČ£¬ĘäČ¼ĮĻÓŠµÄŹĒÖĘ×÷³ÉŠĶµÄ”°·äĪŃĆŗ”±£®
ÓŠŠ©µŲ·½ČĖĆĒ³£ÓĆĆŗĀÆĄ“ÉÕĖ®”¢Č”ÅÆµČ£¬ĘäČ¼ĮĻÓŠµÄŹĒÖĘ×÷³ÉŠĶµÄ”°·äĪŃĆŗ”±£® CaSO3£¬2CaSO3+O2=2CaSO4£®·ÖĪöÉĻŹöĮ½øö·“Ó¦æÉÖŖ£¬ÖĘ×÷”°·äĪŃĆŗ”±Ź±¼ÓČėÉśŹÆ»ŅµÄ×÷ÓĆŹĒ£ŗ________£®
CaSO3£¬2CaSO3+O2=2CaSO4£®·ÖĪöÉĻŹöĮ½øö·“Ó¦æÉÖŖ£¬ÖĘ×÷”°·äĪŃĆŗ”±Ź±¼ÓČėÉśŹÆ»ŅµÄ×÷ÓĆŹĒ£ŗ________£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗŌĘÄĻŹ”ĘŚÄ©Ģā ĢāŠĶ£ŗĢīæÕĢā
 CaSO3£¬2CaSO3+O2=2CaSO4£®·ÖĪöÉĻŹöĮ½øö·“Ó¦æÉÖŖ£¬ÖĘ×÷”°·äĪŃĆŗ”±Ź±¼ÓČėÉśŹÆ»ŅµÄ×÷ÓĆŹĒ£ŗ _______________________ £®
CaSO3£¬2CaSO3+O2=2CaSO4£®·ÖĪöÉĻŹöĮ½øö·“Ó¦æÉÖŖ£¬ÖĘ×÷”°·äĪŃĆŗ”±Ź±¼ÓČėÉśŹÆ»ŅµÄ×÷ÓĆŹĒ£ŗ _______________________ £®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğŌĘÄĻŹ”µĀŗźÖŻĀ¤“ØĻŲ¾ÅÄź¼¶£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
 CaSO3£¬2CaSO3+O2=2CaSO4£®·ÖĪöÉĻŹöĮ½øö·“Ó¦æÉÖŖ£¬ÖĘ×÷”°·äĪŃĆŗ”±Ź±¼ÓČėÉśŹÆ»ŅµÄ×÷ÓĆŹĒ£ŗ £®
CaSO3£¬2CaSO3+O2=2CaSO4£®·ÖĪöÉĻŹöĮ½øö·“Ó¦æÉÖŖ£¬ÖĘ×÷”°·äĪŃĆŗ”±Ź±¼ÓČėÉśŹÆ»ŅµÄ×÷ÓĆŹĒ£ŗ £®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com